Crucial Steps for Singapore Medical Device Registration & HSA Approval

Discover the crucial steps for successful Singapore medical device registration and HSA approval. Operon Strategist offers expert guidance, classification insights, and comprehensive support. Contact Operon Strategist to learn more and navigate the regulatory landscape with confidence.

Singapore Medical Device Regulatory Process

Credevo

Regulatory Oversight of Cell, Tissue, and Gene Therapy Products in Singapore

Singapore's HSA - Global Regulatory Partners, Inc.

Kallol Sen on LinkedIn: #medicaldevices #invitrodiagnostics #productregistration
Singapore Prepares UDI System, Malaysia Seeks Regulatory Improvements And Indonesia Adjusts To Changing Environment :: Medtech Insight

Medical Device Registration in Singapore – Pre-submission Meetings with Health Sciences Authority

Crucial Steps for Singapore Medical Device Registration & HSA Approval
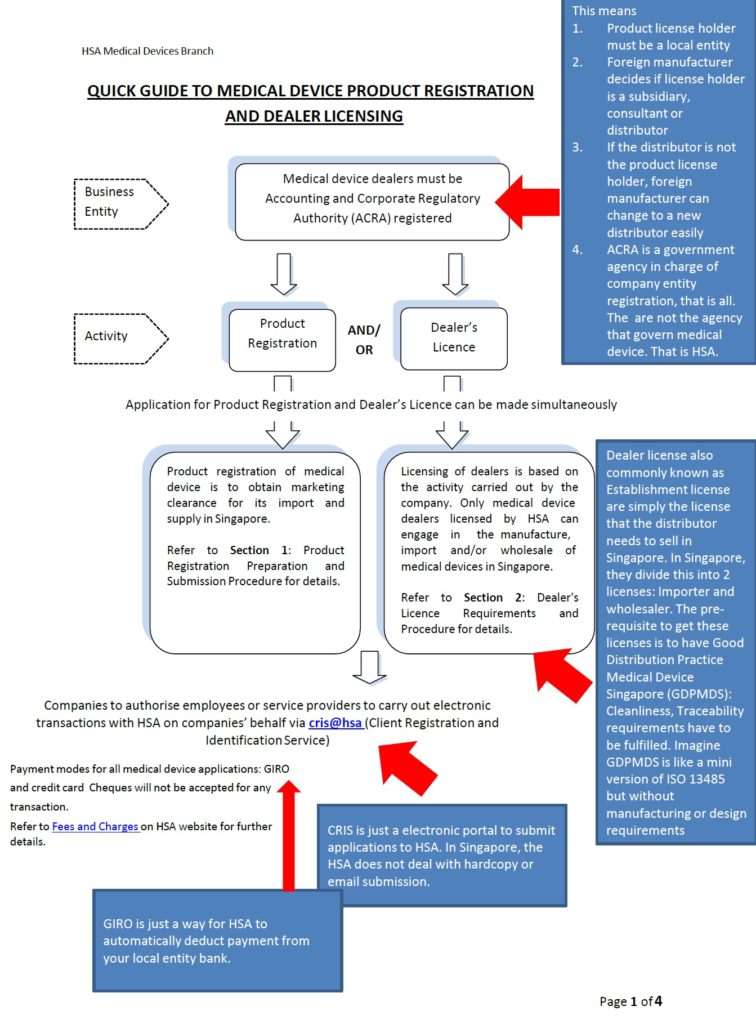
Medical Device registration in Singapore, Health Sciences Authority

HSA Guidance on Medical Device Product Registration: Additional Aspects
Novavax gets Singapore's HSA approval for prototype Nuvaxovid
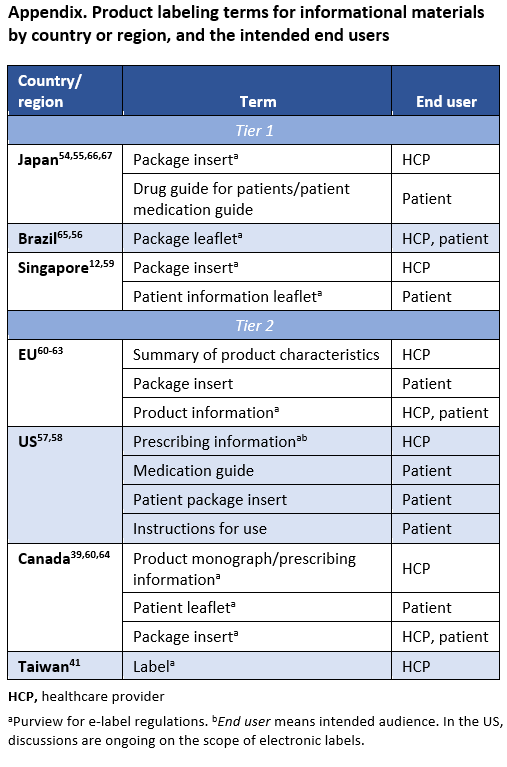
E-labeling and digital transformation in healthcare