10.9: Real Gases - Deviations from Ideal Behavior - Chemistry LibreTexts
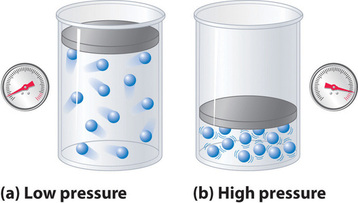
No real gas exhibits ideal gas behavior, although many real gases approximate it over a range of conditions. Gases most closely approximate ideal gas behavior at high temperatures and low pressures. …
No real gas exhibits ideal gas behavior, although many real gases approximate it over a range of conditions. Gases most closely approximate ideal gas behavior at high temperatures and low pressures. Deviations from ideal gas law behavior can be described by the van der Waals equation, which includes empirical constants to correct for the actual volume of the gaseous molecules and quantify the reduction in pressure due to intermolecular attractive forces.

Reporte practica 5 1 .docx - Gases Ideales Ana María Toscano & Esteban Gonzalez Universidad de los Andes. Email: a.gomezt uniandes.edu.co

3.5 Real Gases: Deviation from Ideal

Real gases: Deviations from ideal behavior, AP Chemistry

Real gases: Deviations from ideal behavior, AP Chemistry

Chapter 10 - Gases Prof. Dr. Nizam M. El-Ashgar - ppt download

George Mason University General Chemistry 211 Chapter 5 - ppt download

Real gases: Deviations from ideal behavior, AP Chemistry

Chapter 6 Gases. - ppt download
What volume will 2.5mol of a gas occupy at 283K and at a pressure of 300torr under ideal conditions? (He also says 3 significant digits and ' (R=62.36L * torr/ (mol *
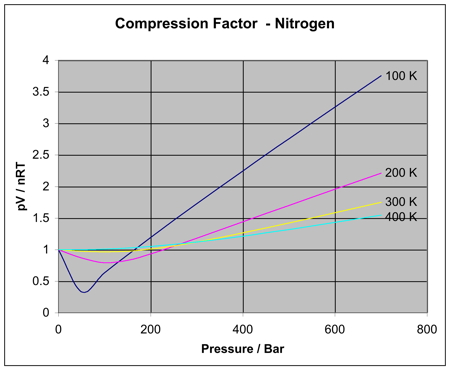
Non-ideal behavior of gases (article)

Chemistry 103-Principles of Chemistry PDF, PDF, Chemical Compounds

Modeling of H2S solubility in ionic liquids using deep learning: A chemical structure-based approach - ScienceDirect