The Cottrell Experiment and Diffusion Limitation 3/3

In this chapter the electrochemical double layer and its features are discussed. The electrochemical double layer acts as a capacitor and every change in the potential of the electrode will induce a capacitive charging current that is caused by physics not by a chemical reaction. This current decays exponentially.

An insight into polyscopoletin electrosynthesis by a quality-by-design approach

Basic potential step and sweep methods

Conventional representation of the Cottrell diffusion ͑ current, I ( t

Chemosensors, Free Full-Text

The Cottrell Experiment and Diffusion Limitation 3/3
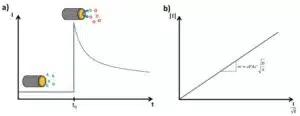
Figure 1.1: Cottrell experiment in KCl solution with

Phase Transformation Lecture 3

Alternative representation of the Cottrell diffusion according to
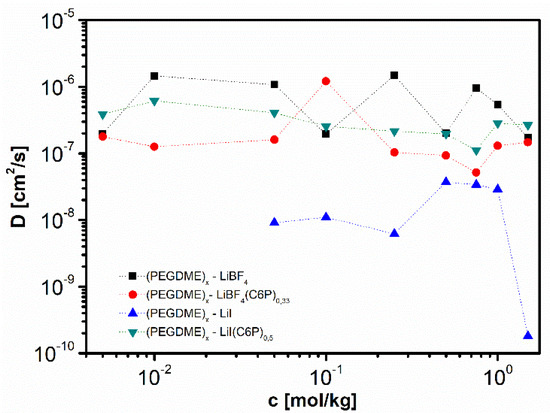
Polymers, Free Full-Text
The Cottrell Experiment and Diffusion Limitation 2/3 - The Cottrell Experiment - PalmSens
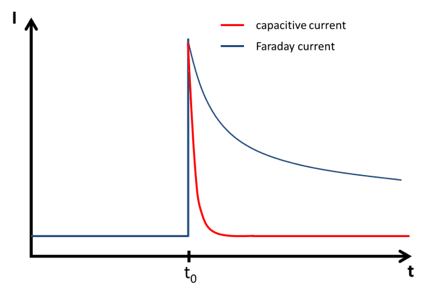
Capacitive Current - PalmSens

5 Mass transport (*diffusion, Fick's laws, Cottrell equation, Nernst diffusion layer)
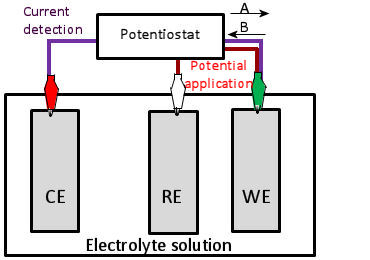
support/electrochemical technique
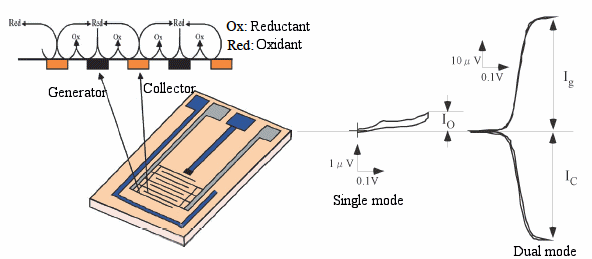
EC_electrode_handbook ALS,the electrochemical company