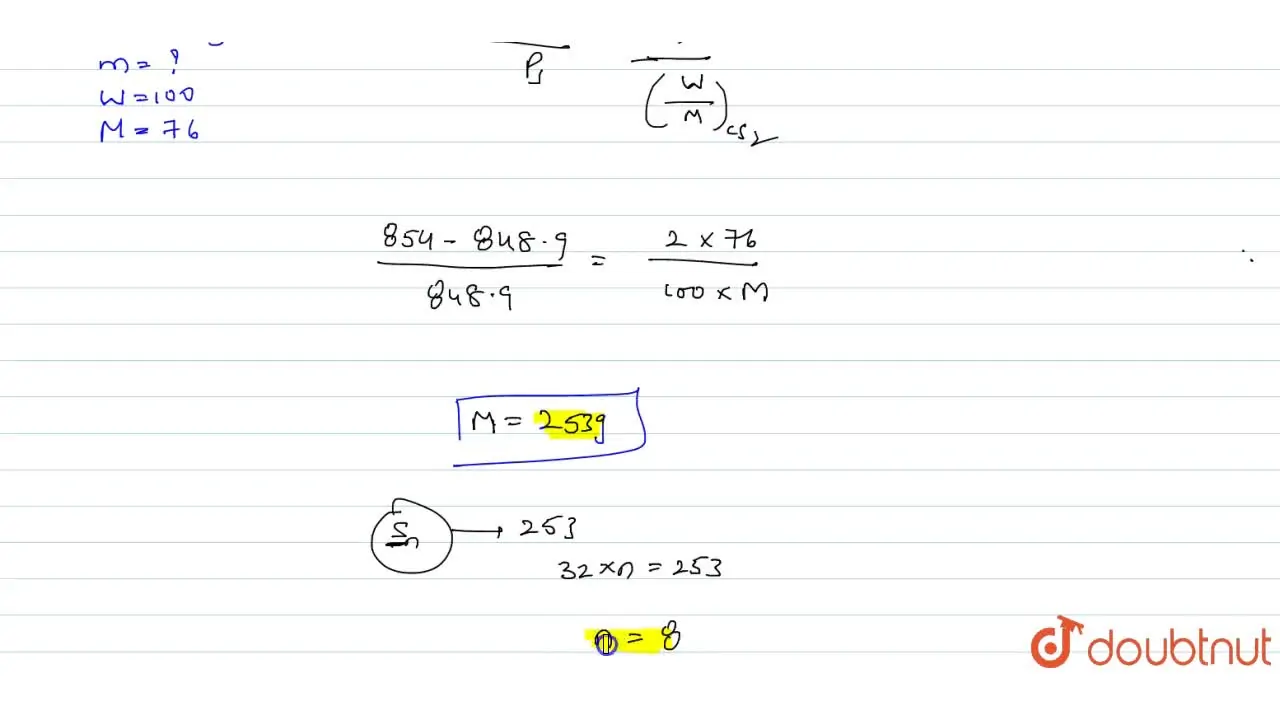
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1)
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1) X 2)X2 3)X4 4)X8
The vapour pressure of a solution having 2-0 g of solute X -gram atomic mass-32 g-mol- in 100 g of CS2 -vapour pressure -854torr- is 848-9 torr-The molecular formula of solute 1- X 2-X2 3-X4 4-X8

The vapour pressure of CS_(2) at 50^(@)C is 854 torr and a solution of 2.0 g sulphur in 100 g of

Solutions (1-47) - Final, PDF, Solubility

The vapour pressure of CS(2) at 50^(@)C is 854 torr and a solution o
How to calculate the vapour pressure lowering caused by the addition of 200g of sucrose (mol mass=342) to 500g of water if the vapor pressure of pure water at 25 degrees Celsius

Solutions (1-47) - Final, PDF, Solubility

Solved 1. Assuming Raoult's Law applies, calculate the vapor

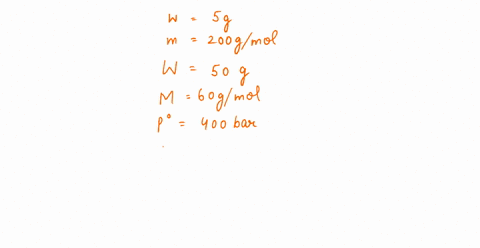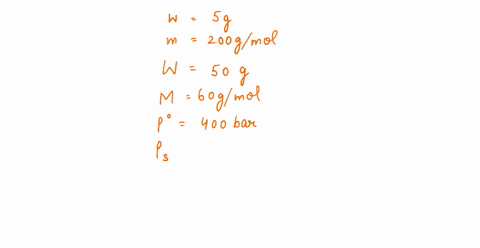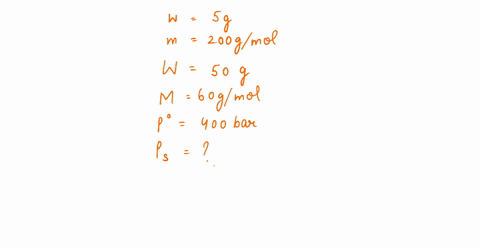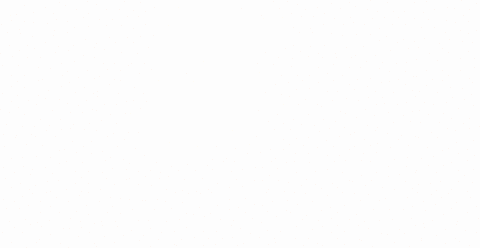
SOLVED: The vapour pressure of a solution having 2.0 g of solute X

16. The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass = 32 g mol-') in 100 g of CS, (vapour pressure = 854 torr) is 848.9
The vapour pressure of CS(2) at 50^(@)C is 854 torr and a solution o

The vapour pressure of a solution having 2.0 g of a solute X( molar mass ..