Typical steady-state polarization curve obtained for a gold electrode

Download scientific diagram | Typical steady-state polarization curve obtained for a gold electrode (length 0.50 cm, width 0.60 cm) in a channel-type cell (halfheight 0.16 cm, width, 1.2 cm) in a 10 mM Na2SO3 in 0.50 M phosphate buffer solution (pH ) 5.25) at a flow rate of 0.32 mL/s, i.e., vo ) 2.5 cm/s (solid circles, right ordinate). The open squares (left ordinate) are the absorbances at 316 nm measured downstream of the electrode as a function of the applied potential. The ordinates were scaled to show the direct proportionality between the current and the amount of dithionite detected spectroscopically in the range-0.50 to-0.75 V (see text for details). The lines represent a polynomial fit to the data. from publication: In Situ Spectroscopic Determination of Faradaic Efficiencies in Systems with Forced Convection under Steady State: Electroreduction of Bisulfite to Dithionite on Gold in an Aqueous Electrolyte | The reduction of bisulfite on Au electrodes in buffered aqueous solutions (pH = 5.25) was examined by in situ near-normal-incidence UV−visible reflection absorption spectroscopy on a rotating disk electrode (RDE) and in situ transmission UV−visible spectroscopy downstream | In Situ, Spectroelectrochemistry and Efficiency | ResearchGate, the professional network for scientists.

Polarization Curve - an overview

Faradaic efficiency for dithionite generation, i.e., jP/j, as a

Microstructural engineering of high-power redox flow battery electrodes via non-solvent induced phase separation - ScienceDirect

Typical steady-state polarization curve obtained for a gold electrode
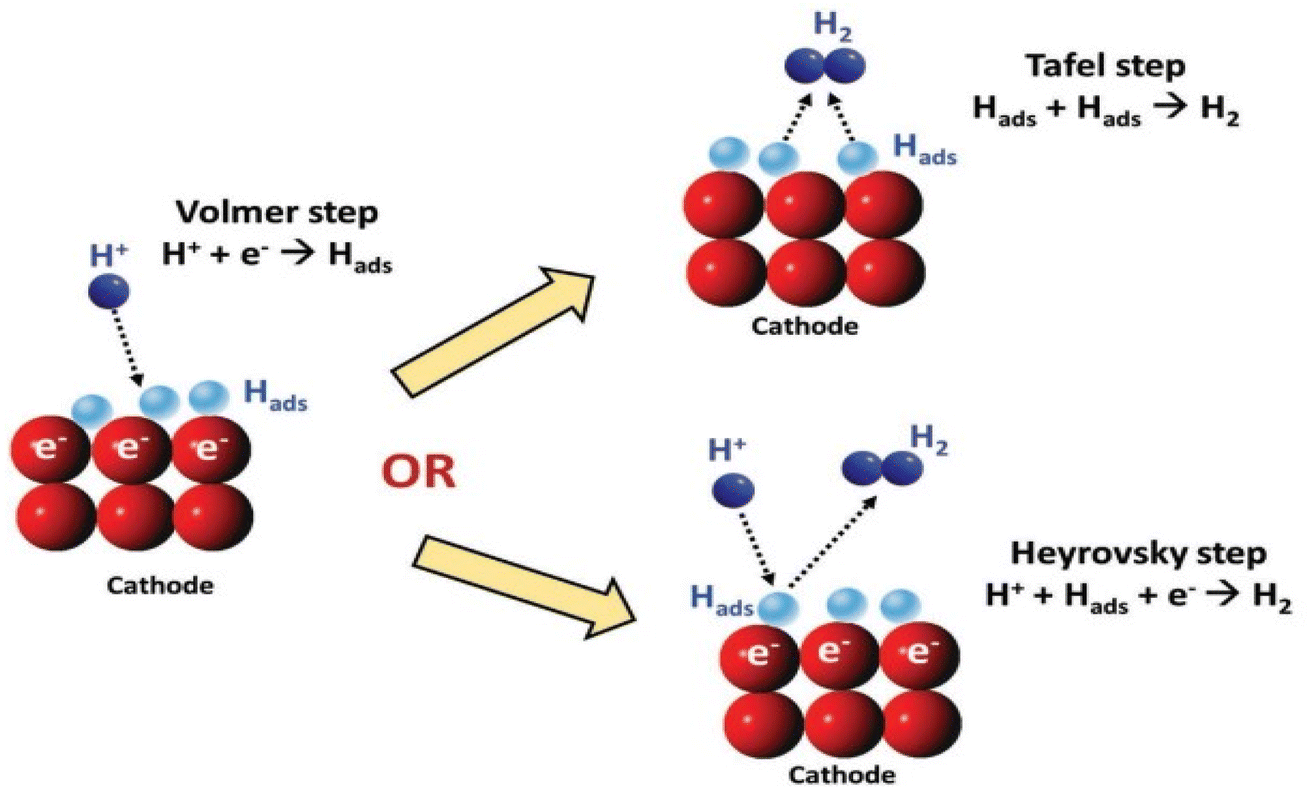
A comprehensive review on the electrochemical parameters and recent material development of electrochemical water splitting electrocatalysts - RSC Advances (RSC Publishing) DOI:10.1039/D2RA07642J

Selective electrochemical reduction of mercury(II) from a simulated traditional gold mining wastewater contaminated with cyanide and heavy metals

Electrochem Eng L03-08 Polarization curve and example for an electrode reaction
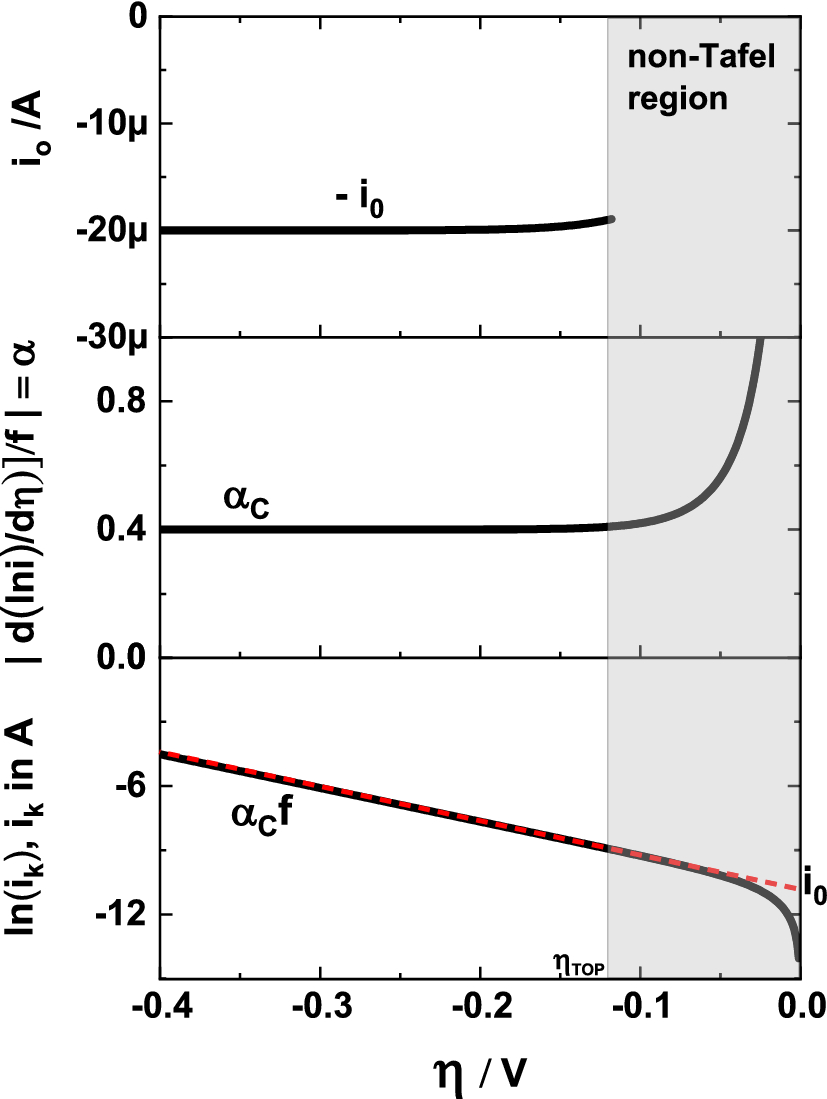
A simple and effective method for the accurate extraction of kinetic parameters using differential Tafel plots
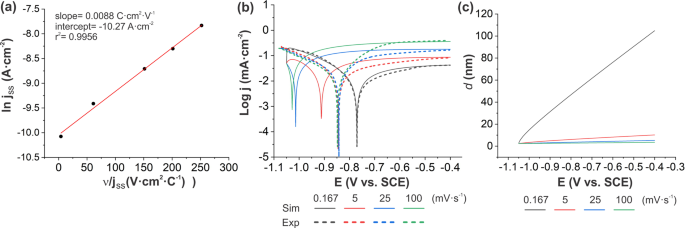
Potentiodynamic polarization curves of AA7075 at high scan rates interpreted using the high field model

Averaged polarization curves of 30 cm² active area Nafion® 117 based

PDF) In Situ Spectroscopic Determination of Faradaic Efficiencies

Overpotential vs. time curve for plating in SPS with other

Polarization curves for the ORR on Pt(poly) obtained at different

PDF) In Situ Spectroscopic Determination of Faradaic Efficiencies