Useful Forms – UK NEQAS – ICC & ISH
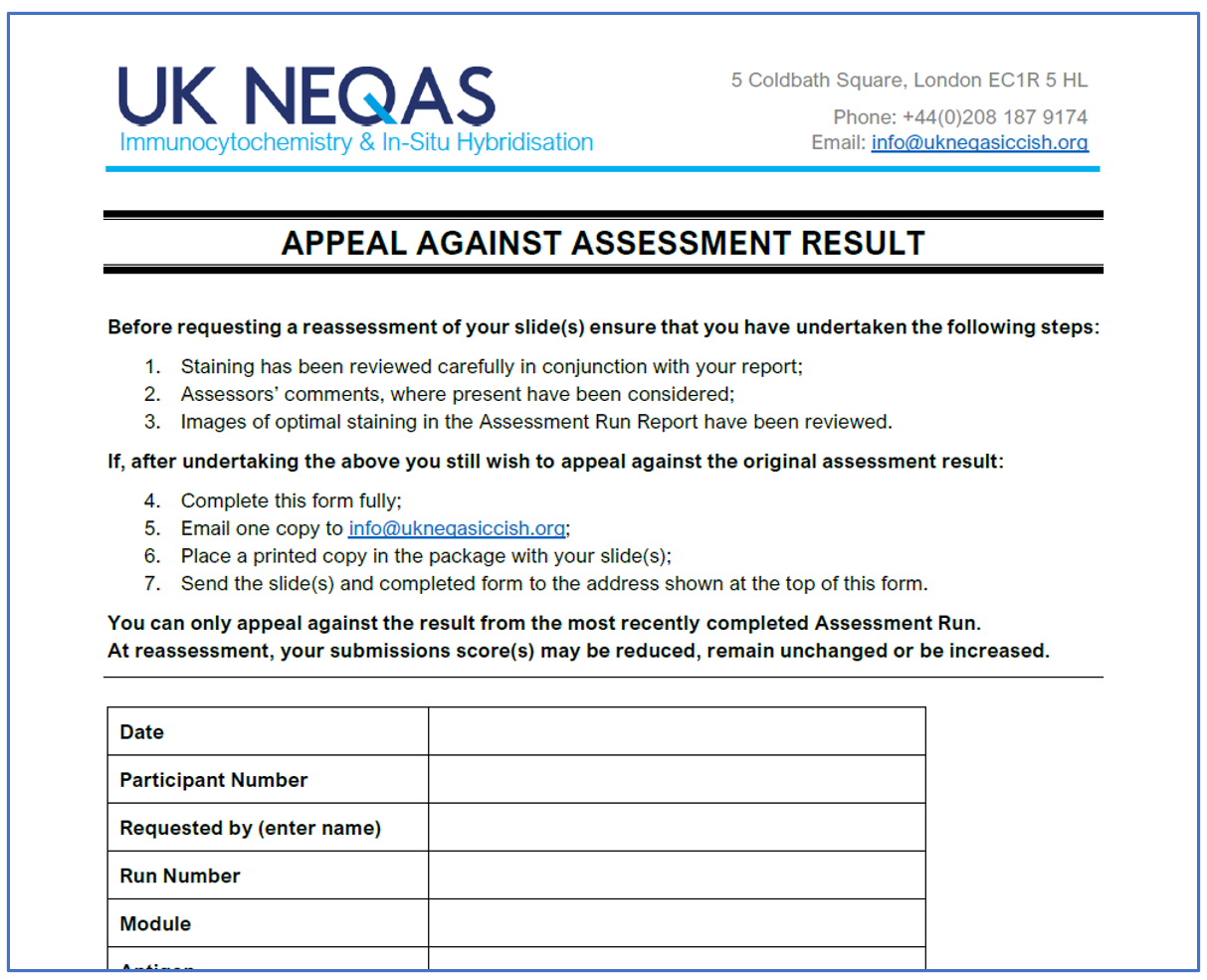

Participants' Manual & Participants' Quick Guide – UK NEQAS – ICC & ISH

Pathologic Evaluation of Unknown Primary Cancer - ScienceDirect

Improvement in the quality of molecular analysis of EGFR in non-small-cell lung cancer detected by three rounds of external quality assessment
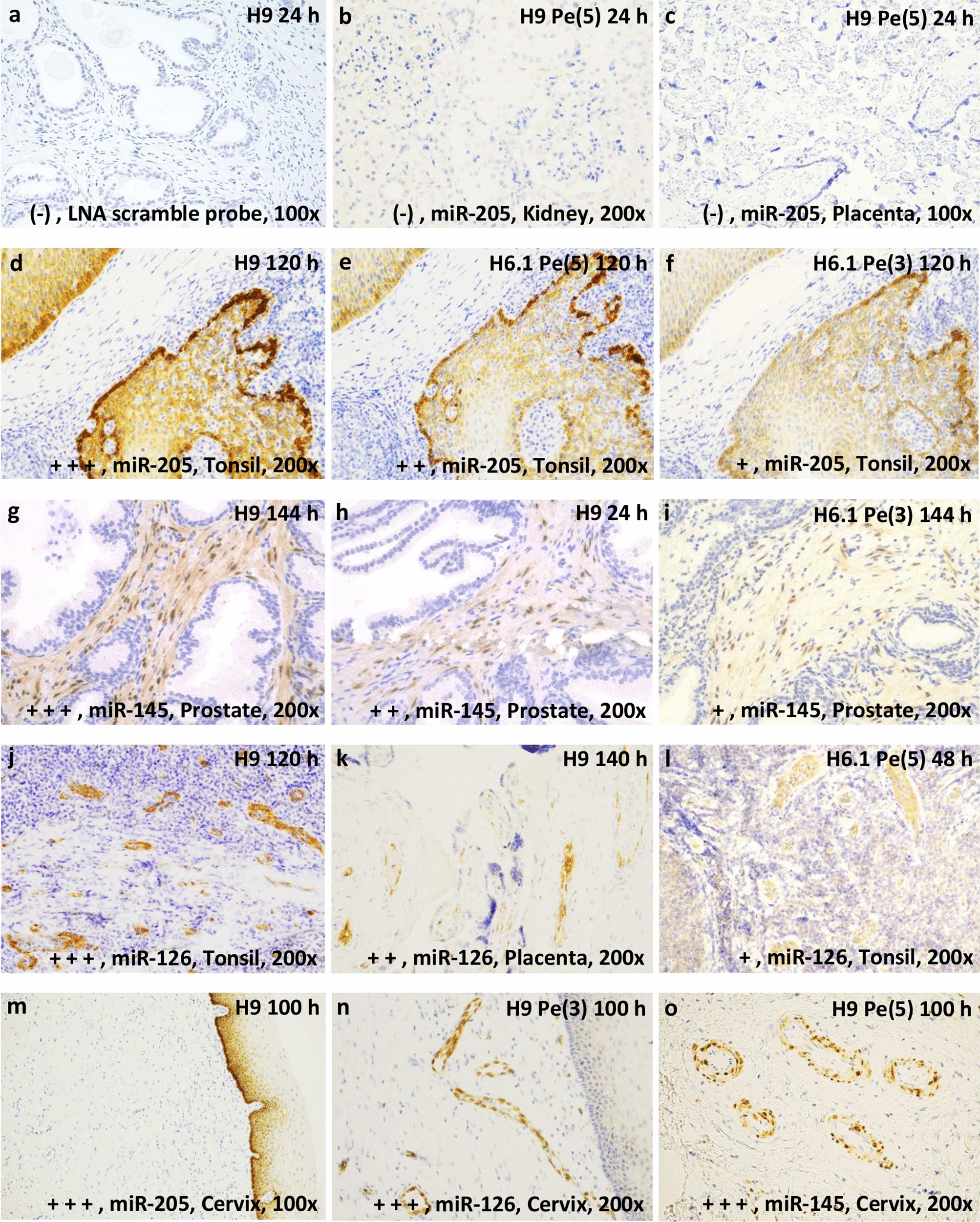
A novel approach for microRNA in situ hybridization using locked nucleic acid probes

Global Ring Study to Investigate the Comparability of Total Assay Performance of Commercial Claudin 18 Antibodies for Evaluation in Gastric Cancer - Laboratory Investigation
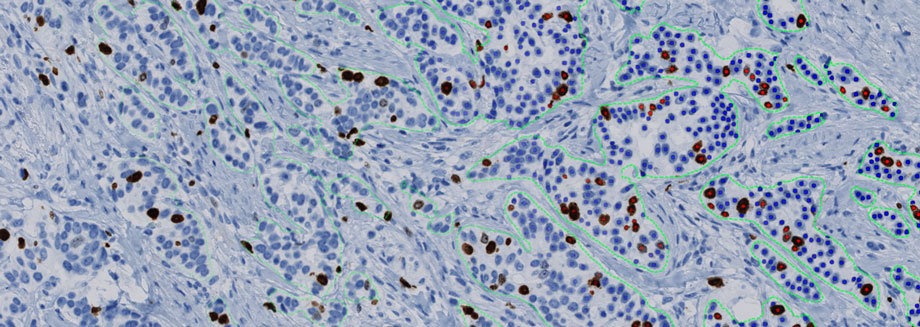
Minerva Imaging and Visiopharm announce collaboration on AI-based image analysis - Visiopharm

e-Journal – UK NEQAS – ICC & ISH

Updated UK Recommendations for HER2 assessment in breast cancer. - Abstract - Europe PMC

PDF) Quality assurance guidance for scoring and reporting for pathologists and laboratories undertaking clinical trial work: Quality assurance in clinical trials

UK NEQAS IQN Path

Assessment Procedure – UK NEQAS – ICC & ISH

UK NEQAS for Immunocytochemistry & In Situ Hybridisation

ERBB2 mutation is associated with sustained tumor cell proliferation after short-term preoperative endocrine therapy in early lobular breast cancer - Modern Pathology

Global Ring Study to Investigate the Comparability of Total Assay Performance of Commercial Claudin 18 Antibodies for Evaluation in Gastric Cancer - Laboratory Investigation
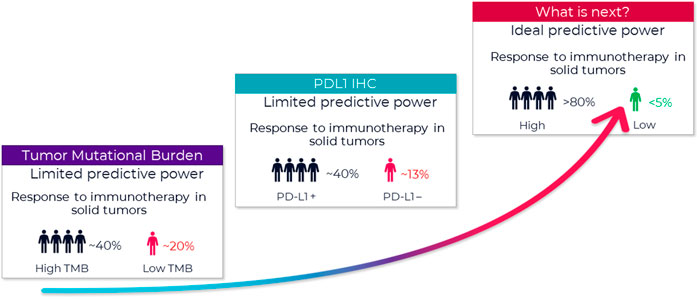
Frontiers Companion diagnostic requirements for spatial biology using multiplex immunofluorescence and multispectral imaging