physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

In the above graph,the minima of the curve for methane is more than that of nitrogen. Also, for a given value of pressure, the value of $Z$ for methane is less than that of nitrogen. They seem to m

Non-Ideal Gas Behavior Chemistry: Atoms First
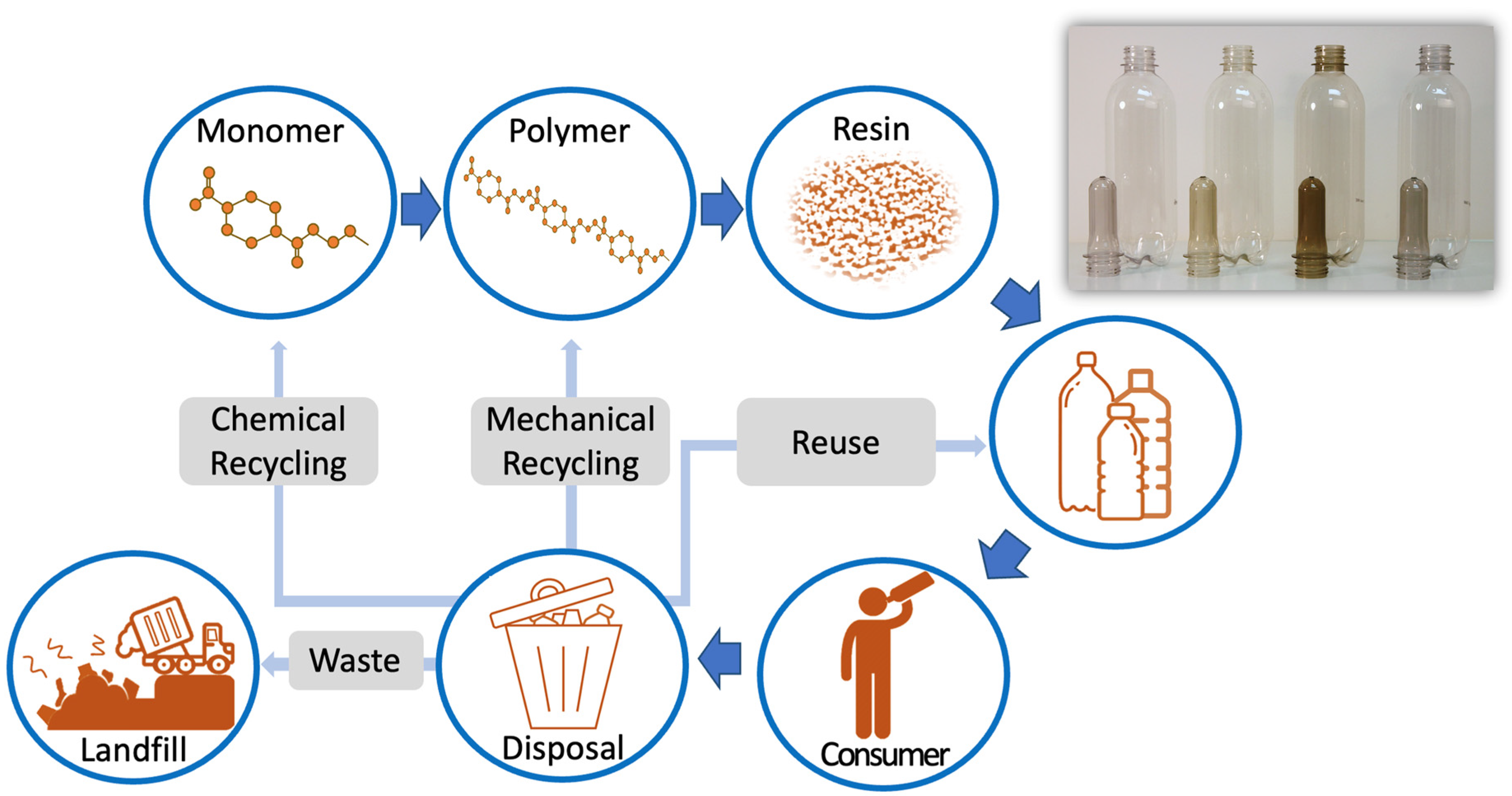
Polymers, Free Full-Text

Review of the Decomposition of Ammonia to Generate Hydrogen

Gas - Wikipedia
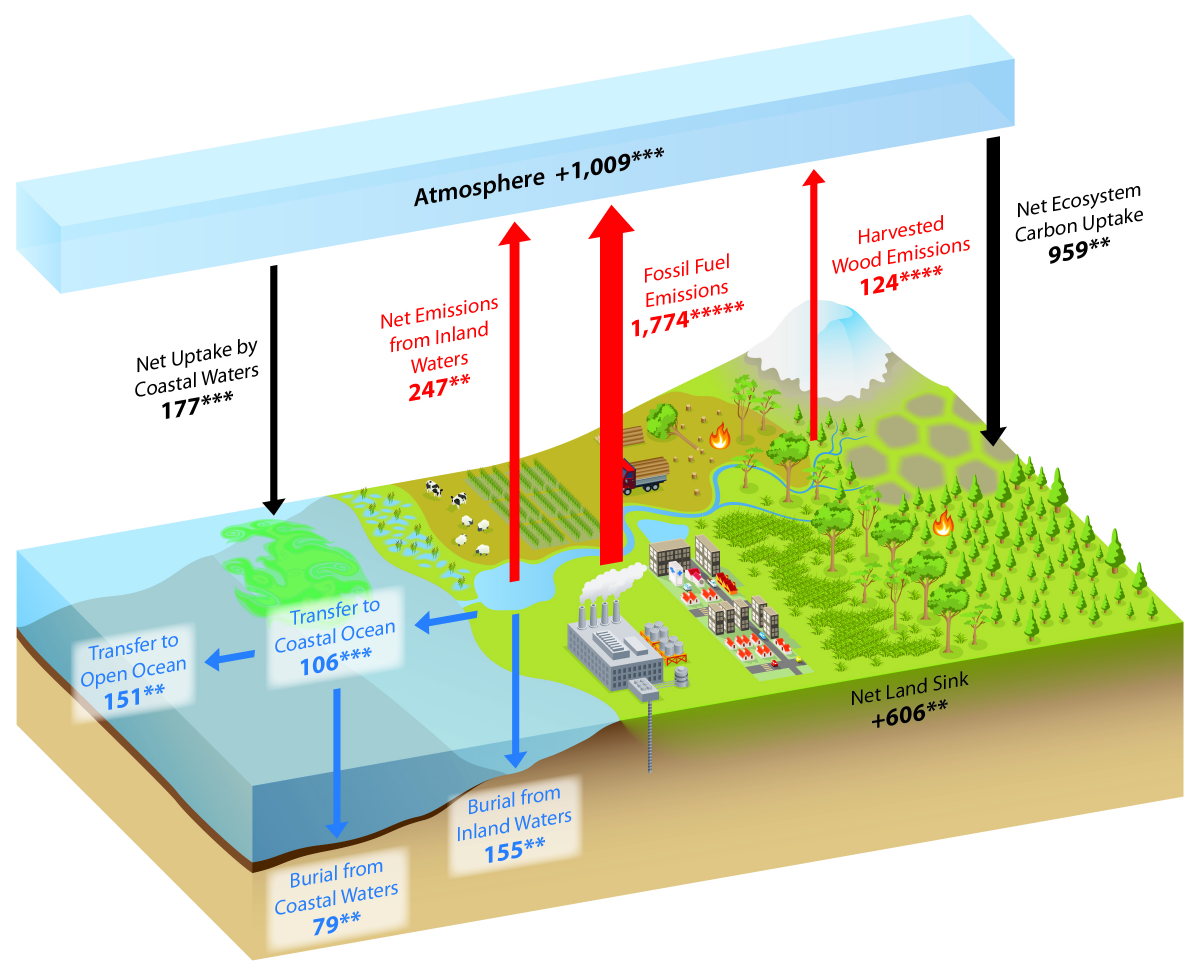
What is the Carbon Cycle? What is the science behind it?

Collision Theory- Definition, Explanation, Activation energy
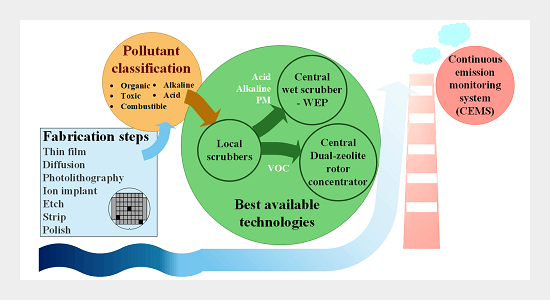
Continuous Improvements and Future Challenges of Air Pollution
How can a gas be ideal at a high pressure and low temperature? - Quora

physical chemistry - Compressibility Factor Graph - Which gas