Write the expression for the compressibility factor (Z) for one mole of a gas. Write the value of Z for an


For H(2) gas, the compressibility factor,Z = PV //n RT is
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect

Selecting the Proper Gas Compressibility Z for Relief Valve Sizing

Ideal Gas vs. Real Gas - Chemistry Review (Video)
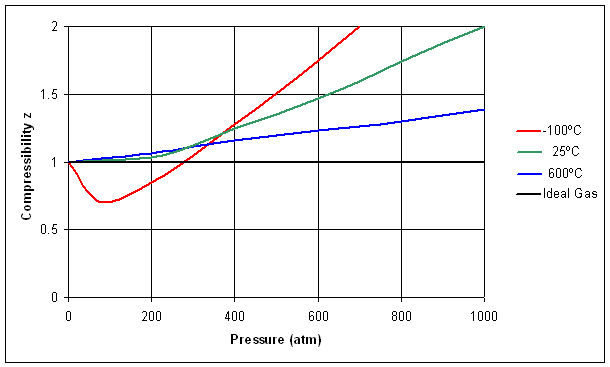
Gas Laws – First Year General Chemistry
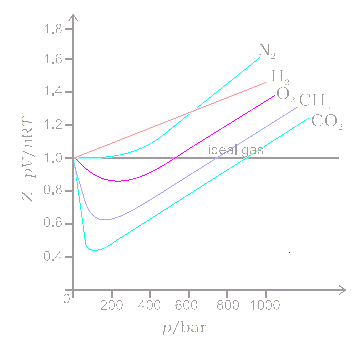
Van der waals equation: Derivation, Explanation

6.3: Van der Waals and Other Gases - Physics LibreTexts

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0

Energies, Free Full-Text

SOLVED: 4.17 The non-ideality of a gas may be expressed as a compressibility factor, z: PVm RT a. Find the value of z for the ideal gas. b. Given the van der

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

SOLVED: 4.17 The non-ideality of a gas may be expressed as a compressibility factor, z: PVm RT a. Find the value of z for the ideal gas. b. Given the van der