For a given gas, a graph is shown between compressibility factor (Z) and Pressure (P).Select the incorrect statement(s) about the various temperature relations.a)Temperature T1 must be above critical temperature (TC).b)Temperature T2 may


compressibility Factor v/s Pressure/ Temperature Graph . States of Matter Class XI.

Solved QUESTION 3 Determine the compressibility

The given graph represents the variation of compressibility factor Z vs P for three gases A, B and C.Identify the incorrect statements.

1.7: Connecting the van der Waals and the viral equations- the Boyle temperature - Chemistry LibreTexts

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange
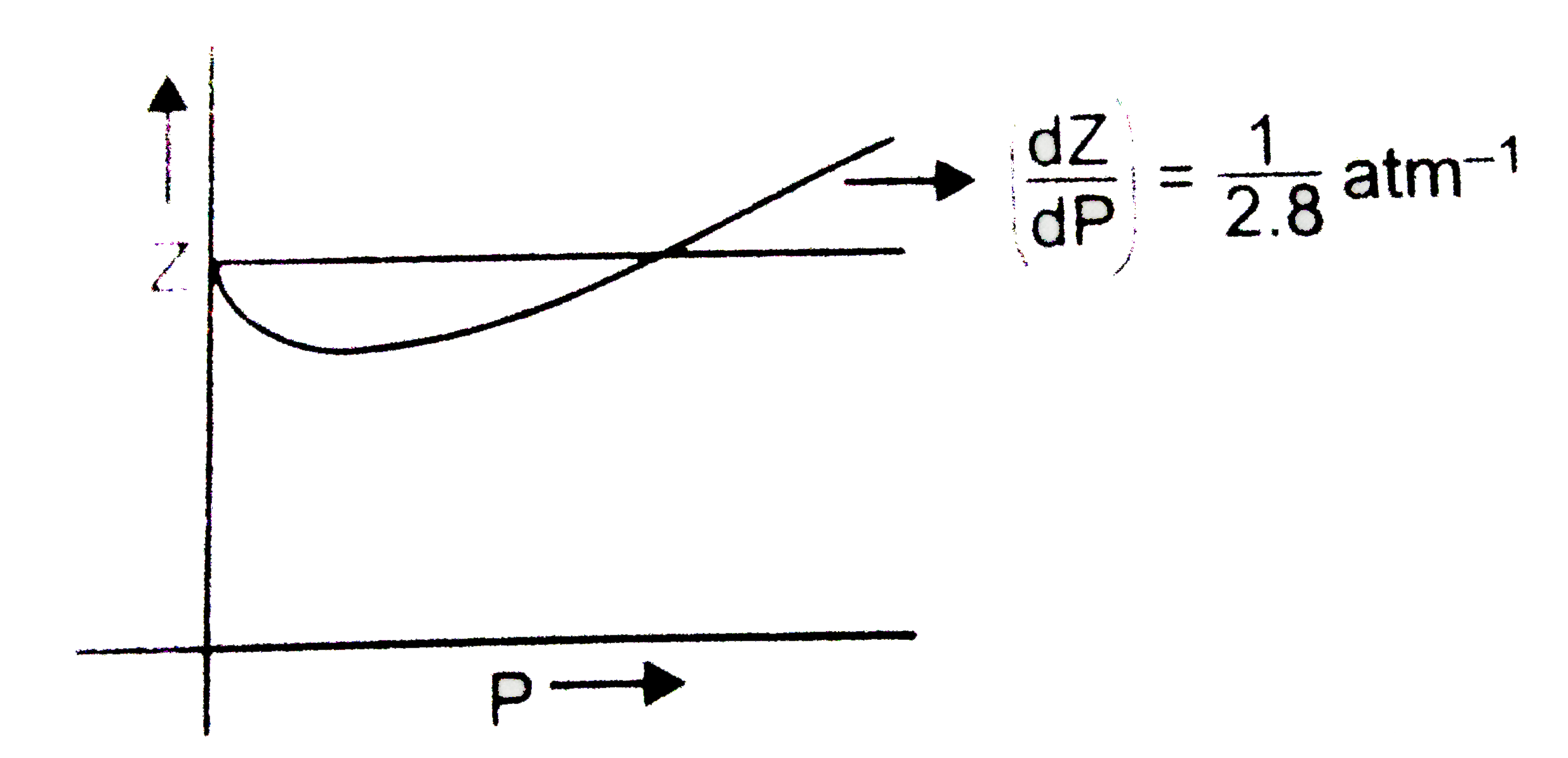
The graph of compressibility factor (Z) vs. P for one mole of a real g

Compressibility factor Z is plotted against pressure P for four different gases A , B , C & D. The correct order of critical temperature of the gases shown in the below

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

PV Compressibility factor Z= nRT is plotted against pressure : N. Ideal gas What is the correct order of liquefiability of the gases shown in the above graph? H

The given graph represents the variation of Z (compressibility factor = \[\dfrac{{PV}}{{nRT}}\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.

compressibility Factor v/s Pressure/ Temperature Graph . States of Matter Class XI.

A graph Z vs P is plotted N_2 gas different temperatureThe correct relationship between temperatures

Compressibility factor - Wikipedia