Aβ(1-42) tetramer and octamer structures reveal edge conductivity

Molecular dynamics simulations reveal the importance of amyloid-beta oligomer β-sheet edge conformations in membrane permeabilization - ScienceDirect

RCSB PDB - 6RHY: Structure of pore-forming amyloid-beta tetramers

RCSB PDB - 6RHY: Structure of pore-forming amyloid-beta tetramers

The amyloid concentric β-barrel hypothesis: Models of amyloid beta 42 oligomers and annular protofibrils
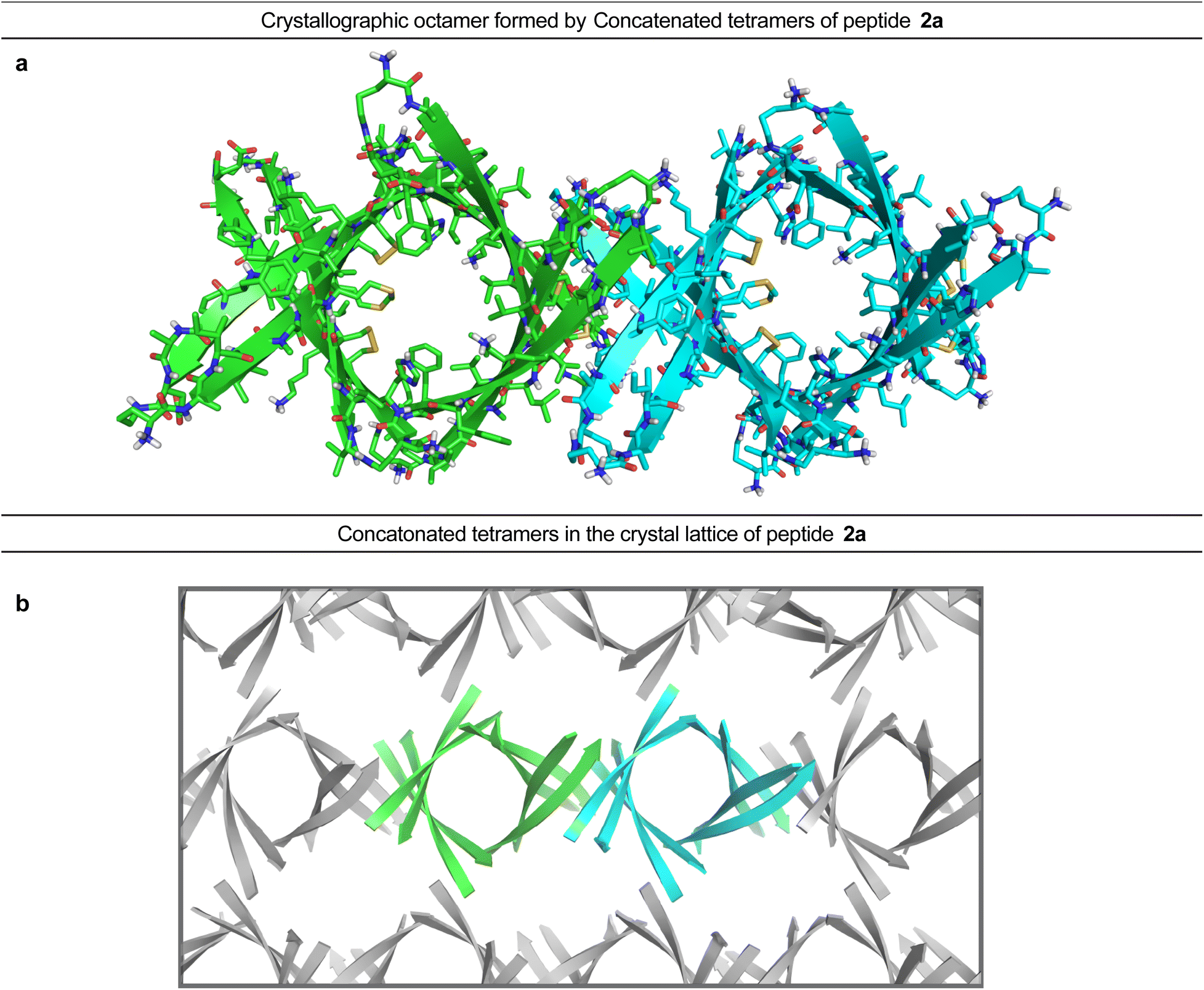
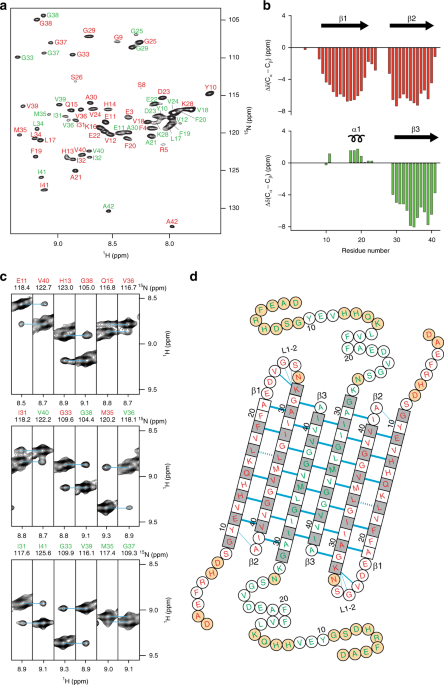
A β-barrel-like tetramer formed by a β-hairpin derived from Aβ

Atomic Structure of Alzheimer's Amyloid Protein Reveals New Toxicity Mechanism
CovalX, Epitope Mapping
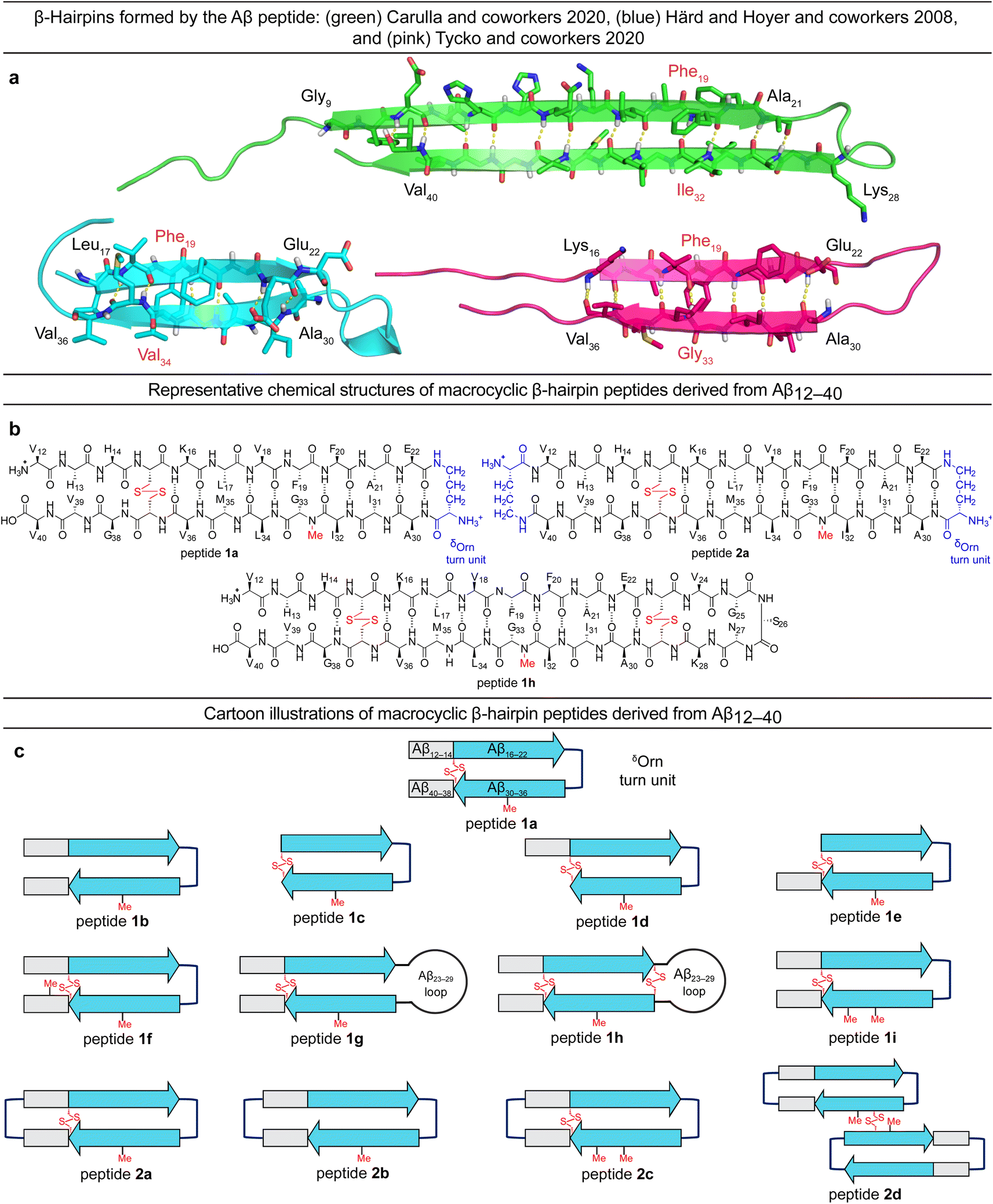
A β-barrel-like tetramer formed by a β-hairpin derived from Aβ - Chemical Science (RSC Publishing) DOI:10.1039/D3SC05185D
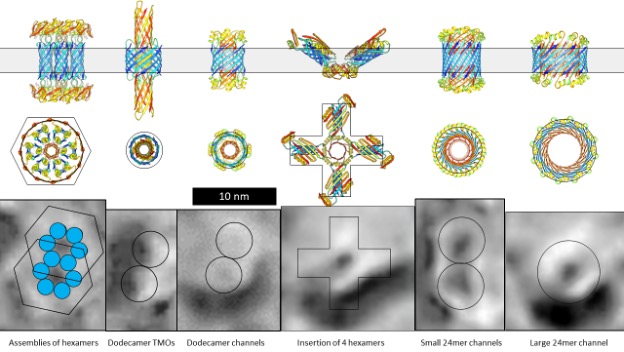
Why are the root causes of amyloid-associated diseases so misunderstood and treatments so inadequate?

The amyloid-inhibiting NCAM-PrP peptide targets Aβ peptide aggregation in membrane-mimetic environments. - Abstract - Europe PMC