thermodynamics - Variation of compressiblity factor with temperature - Chemistry Stack Exchange

While I certainly understand the order of temperatures, I can't find a reason for the curves to intersect at one common point. Why do the curves intersect at one point? or do they really intersect

Liquefaction of Gases - GeeksforGeeks

Hydrogen energy systems: A critical review of technologies, applications, trends and challenges - ScienceDirect
Does specific heat for ideal gas at constant volume depends on temperature? - Quora

Falkovich Statistical - Physics Notes, PDF, Heat

Dehydration kinetics of nanoconfined water in beryl probed by high temperature single crystal synchrotron X-ray diffraction
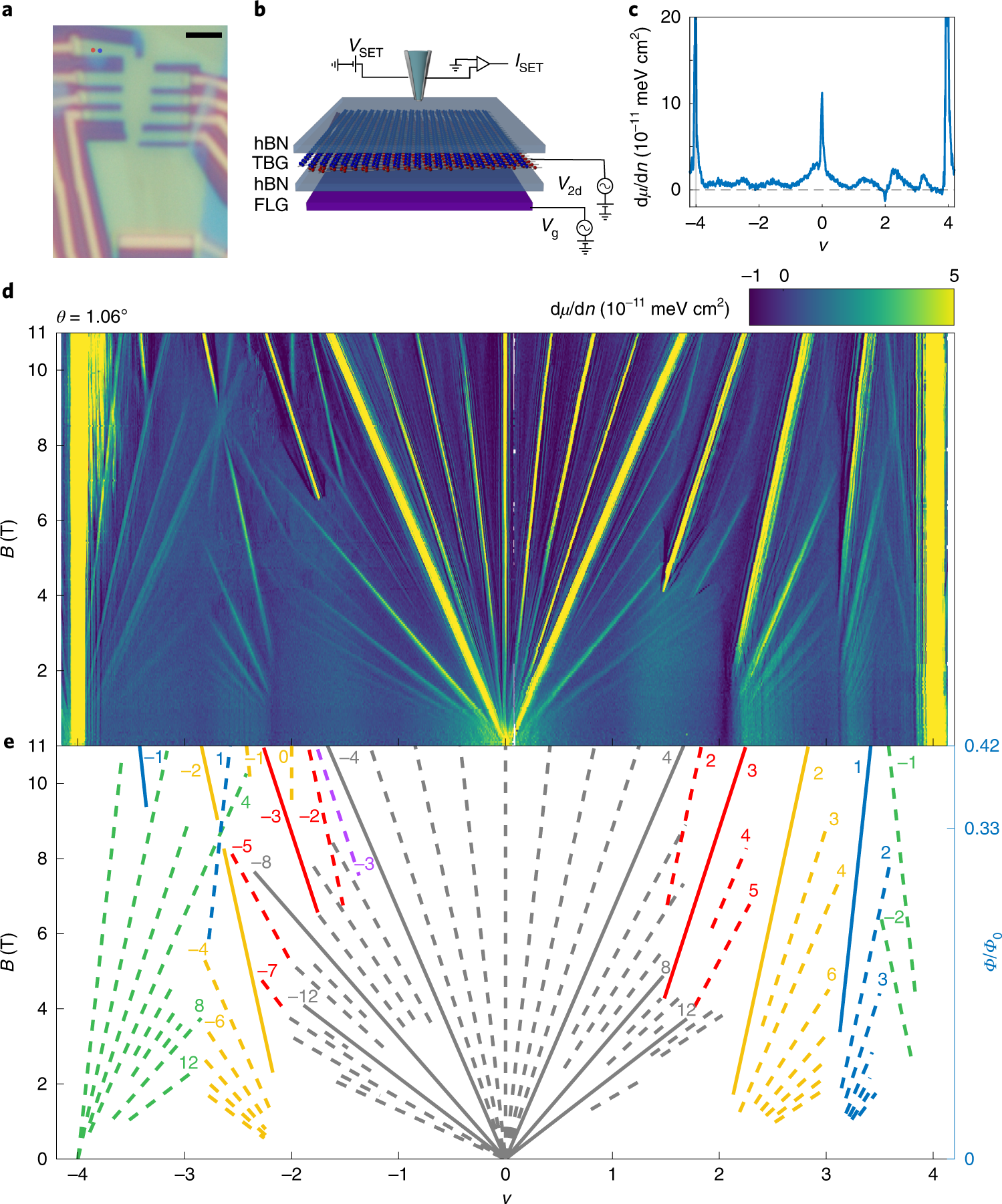
Correlated Hofstadter spectrum and flavour phase diagram in magic-angle twisted bilayer graphene

Entropy, Free Full-Text

Self-preservation and Stability of Methane Hydrates in the Presence of NaCl
OpenKIM · Morse Shifted GirifalcoWeizer 1959LowCutoff Ag MO_137719994600_004 MO_137719994600 · Interatomic Potentials and Force Fields

In the figure representing variation of the compressibility factor Z of a real gas with pressure