kinetic theory - Why doesn't Helium behave as an ideal gas

I am a bit confused (might be due to some conceptual misunderstanding) as to why doesn't Helium behave as an ideal gas (it shows a deviation from the $pV$ vs $p$ graph)? (Given the fact that it is
Is kinetic theory applicable to ideal gas only? If yes, why is it so? - Quora
Why do gases behave ideally? Is there any gas that we can say it

Kinetic Theory: Atomic and Molecular Explanation of Pressure and Temperature

Kinetic Theory of Gases, PDF, Gases

13.4 Kinetic Theory: Atomic and Molecular Explanation of Pressure
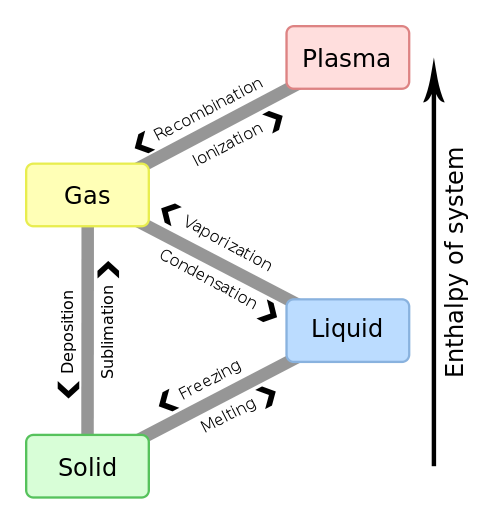
Properties of Gases

The Ideal Gas Law - Video Tutorials & Practice Problems
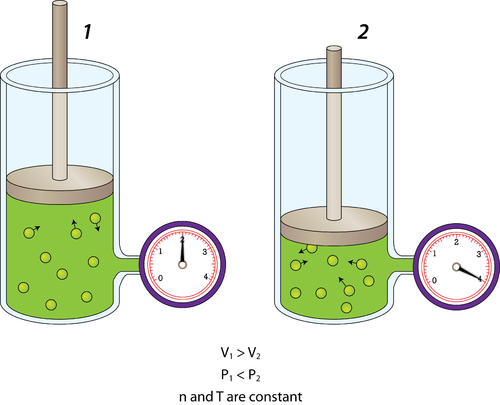
The Behavior of Gases Chemistry for Non-Majors
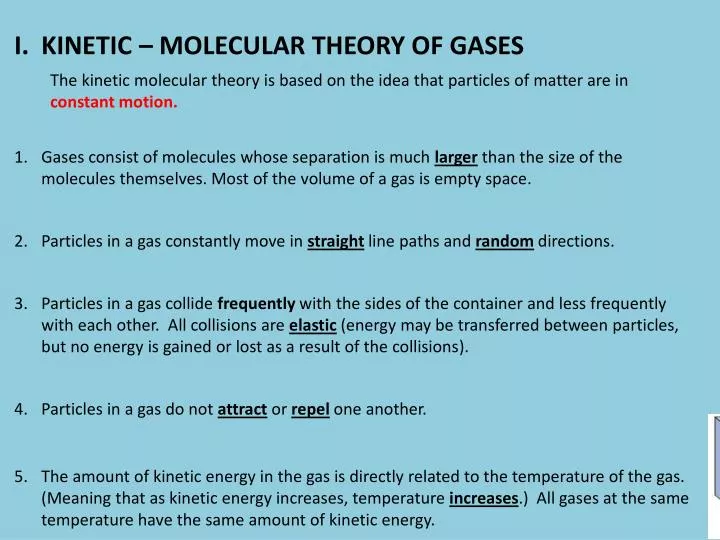
PPT - KINETIC – MOLECULAR THEORY OF GASES PowerPoint Presentation, free download - ID:4176699

Get Answer) - Learning Goal: Kinetic Theory Of Ideal Gas A Monatomic Ideal Gas

NCERT Solutions for Class 11 Physics Chapter 13 Kinetic Theory
qph.cf2.quoracdn.net/main-qimg-f4c2d675e553ef09dc7

Gas - Behaviour, Properties, Physics
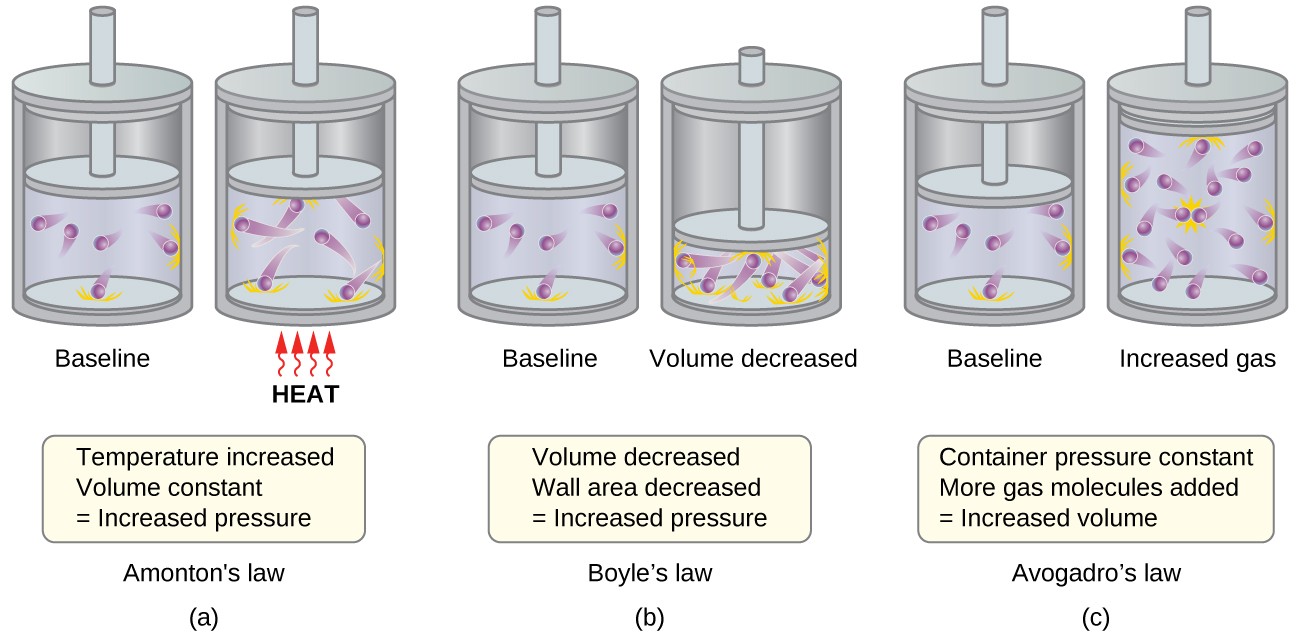
9.5 The Kinetic-Molecular Theory
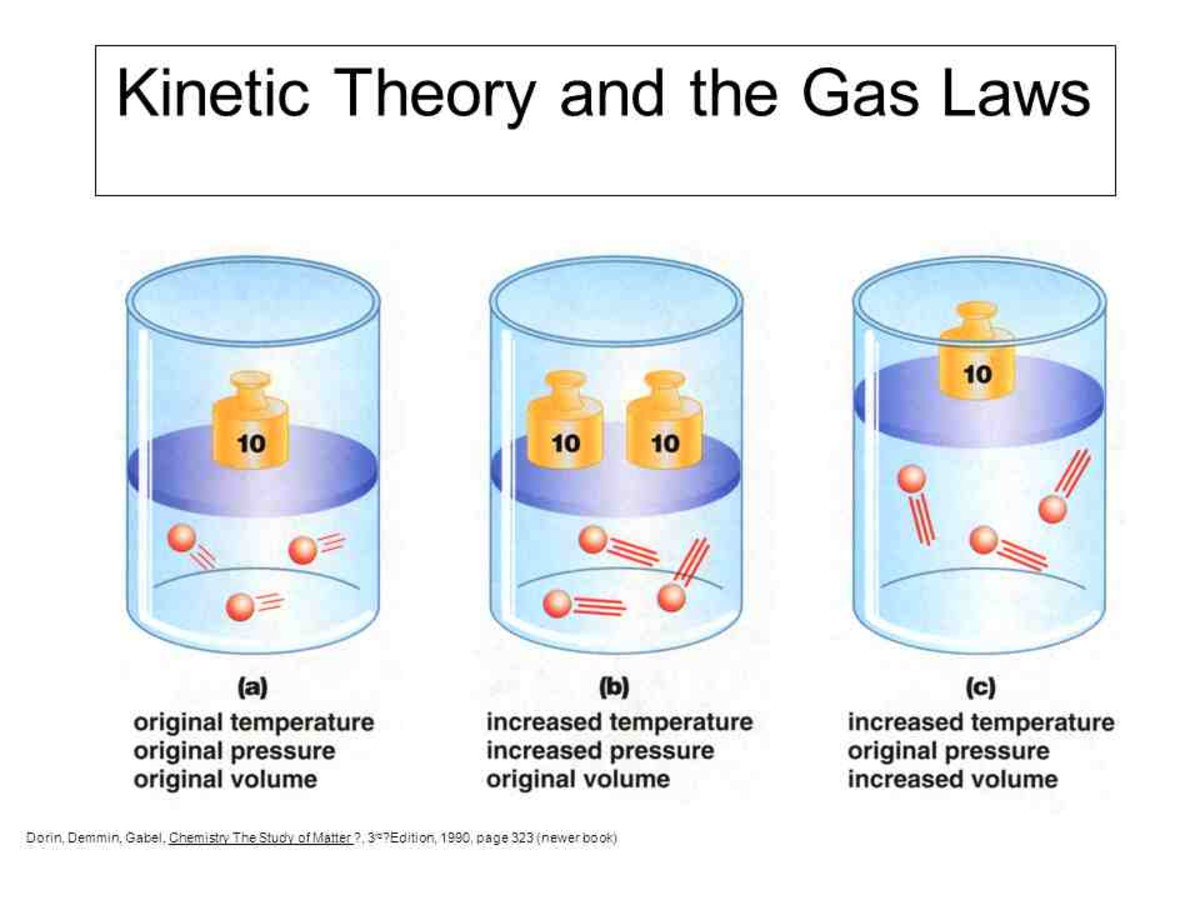
The Theories and Behavior of Gas - Owlcation