42g of N₂ react with excess of O₂ to produce NO. Amount of NO

Share your videos with friends, family, and the world

Mole Concept PDF, PDF, Mole (Unit)

Mole Concept PDF, PDF, Mole (Unit)
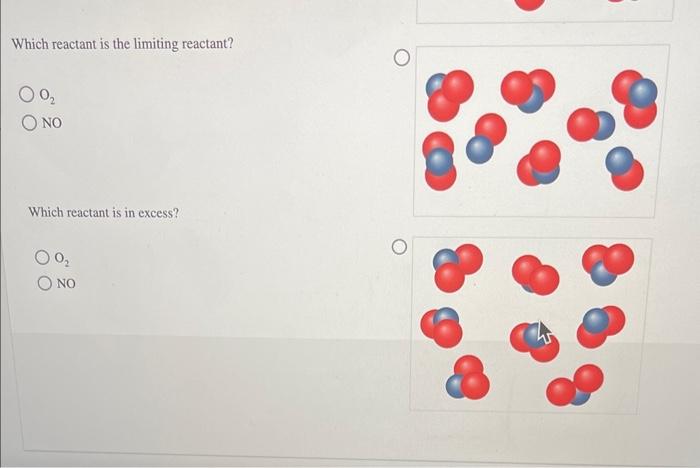
Solved reacts with NO to form NO2 according to the ollowing

6g C react with 22414 cm³ of O₂ to produce CO2. How much non-limiting reactant is in excess. MDCAT

If 10 g of H2. reacts with 42 g of N2, ther identify the correct statem..

Form 3 - Chemistry - Assignment - 237 - 1590689559732-CHEM-F3, PDF, Nitric Acid
How to justify oxidant and reductant in the following reaction N2+O2…2NO - Quora

Answered: 2 NO(g) + O2 (g) –2 NO2(g) What is the…
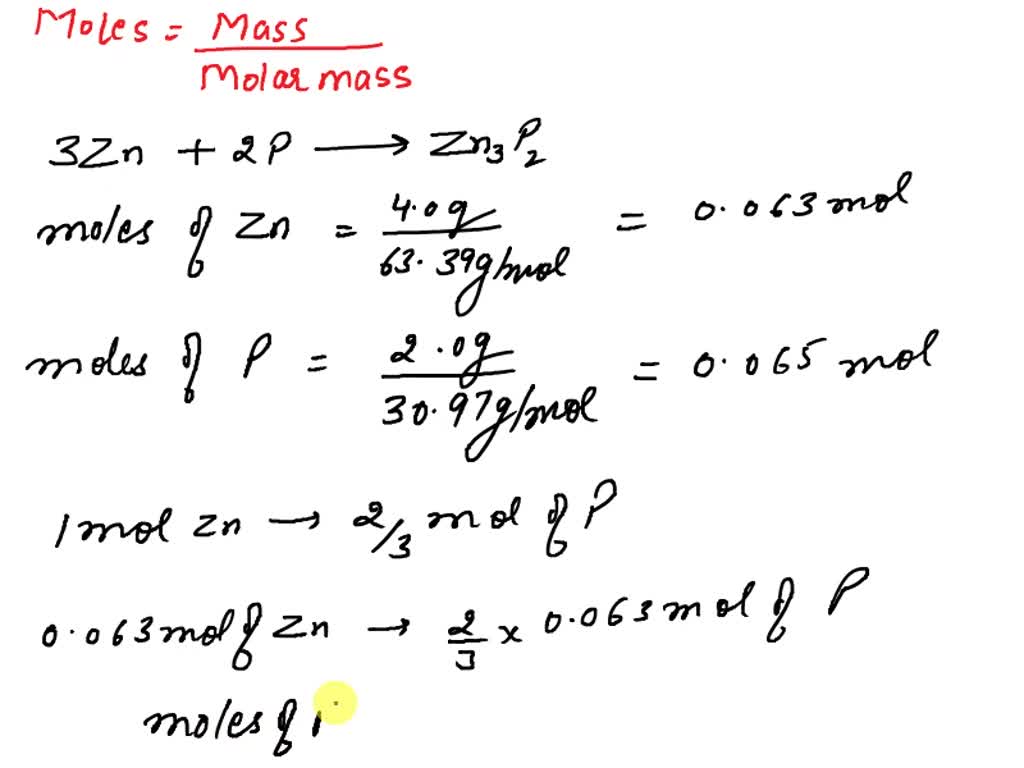
SOLVED: PQ-29. What amount of excess reagent remains when 4.0 g zinc reacts with 2.0 g phosphorus? 3Zn ZnP2 Molar mass Zn = 65.38 g/mol, P = 30.97 g/mol 0.70 g P (
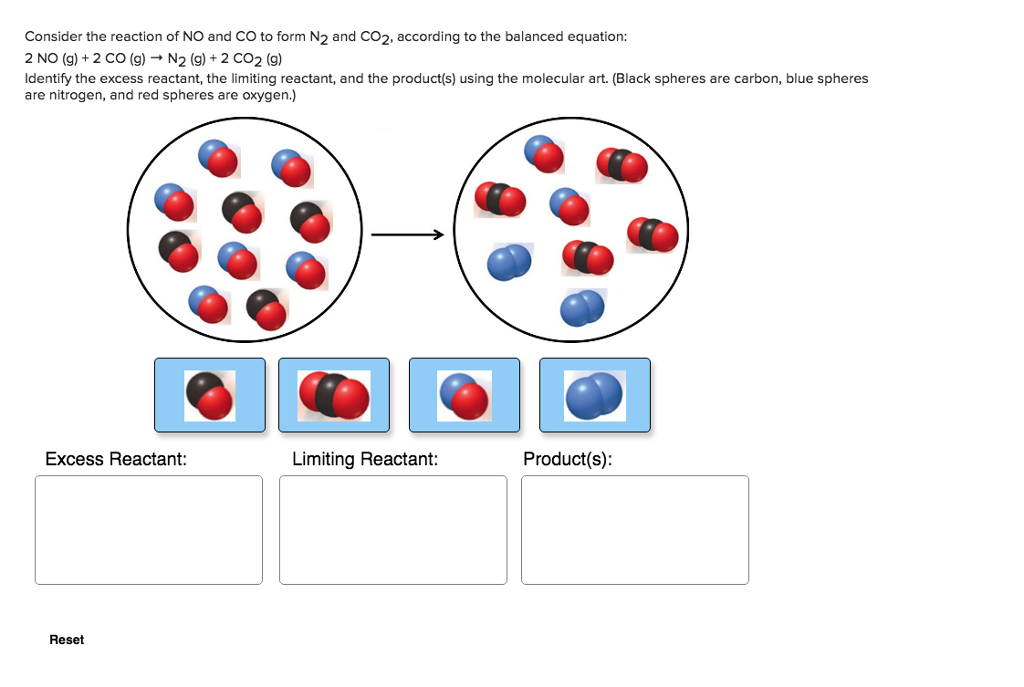
Solved Consider the reaction of NO and CO to form N2 and