the equation of state of a gas is p(v-nb)=rt where b and r are consta - askIITians

the equation of state of a gas is p(v-nb)=rt where b and r are constants. if the pressure and temperature are such that vm=10b what is the value of compressibi

Solved A certain gas obeys the equation of state P V = R T
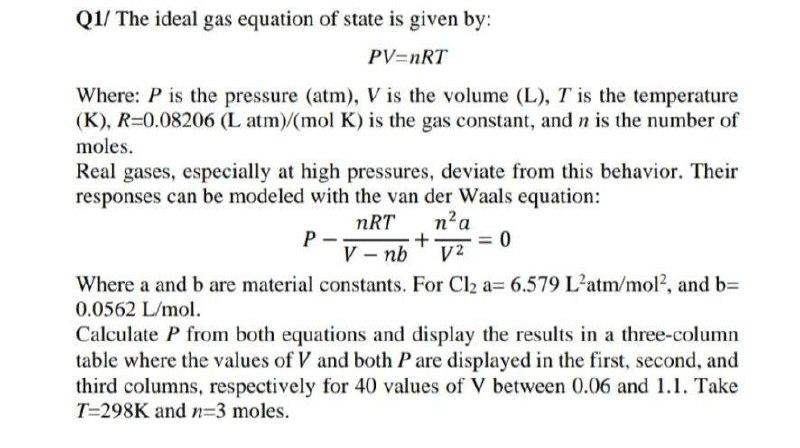
Q1/ The ideal gas equation of state is given by

In the gas equation, (P +dfrac{a}{V^2}) (V - b) = RT , where P is pressure, V is volume, R is gas constant and T is temperature. Calculate the dimensional formula of

The equation of state for real gas is given by (p+a/V2)(V-b)=RT The dimension of the constant a is - Physics - Motion In A Straight Line - 12930723

Gaseous State Notes, PDF, Gases

Deviation From Ideal Gas Behavior - Study Material for IIT JEE


Tutorials For Chemicalthermodynamics, PDF, Chemical Equilibrium
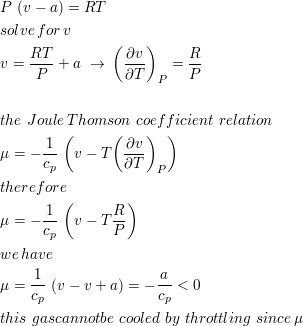
Consider a gas whose equation of state is P(v-a)=RT, where a

Gaseous State Notes, PDF, Gases

The Ideal Gas Law

Solved The Van der Waal's equation of state is: P = nRT/V
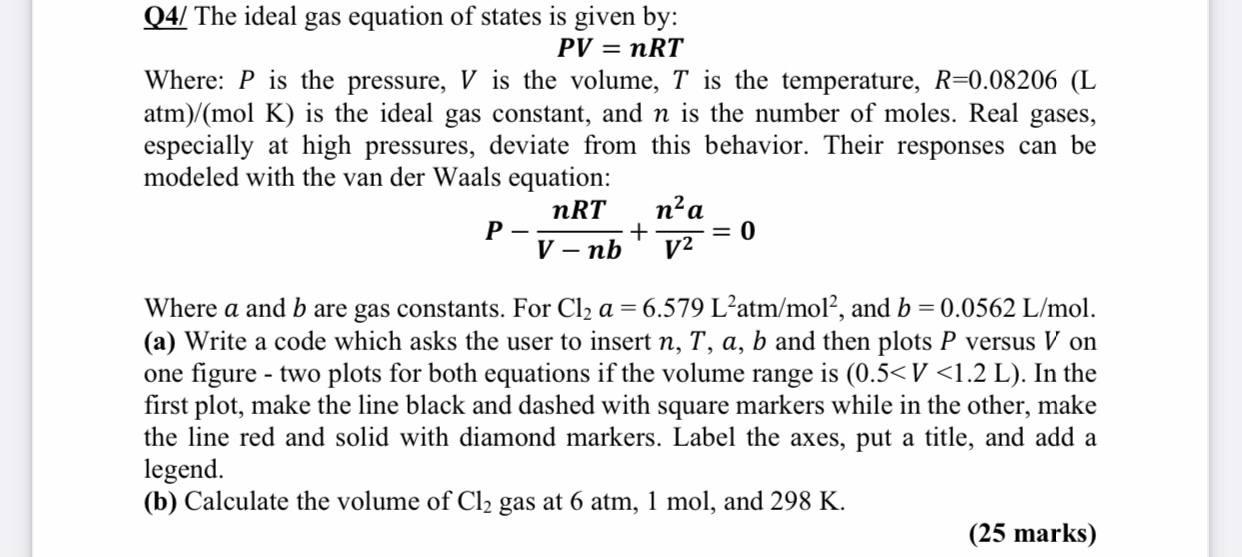
Solved Q4/ The ideal gas equation of states is given by: PV