200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
200 g of a sample of limestone liberates 66 g of CO2 on heating- The percentage purity of CaCO3 in the limestone is Options-a- 95
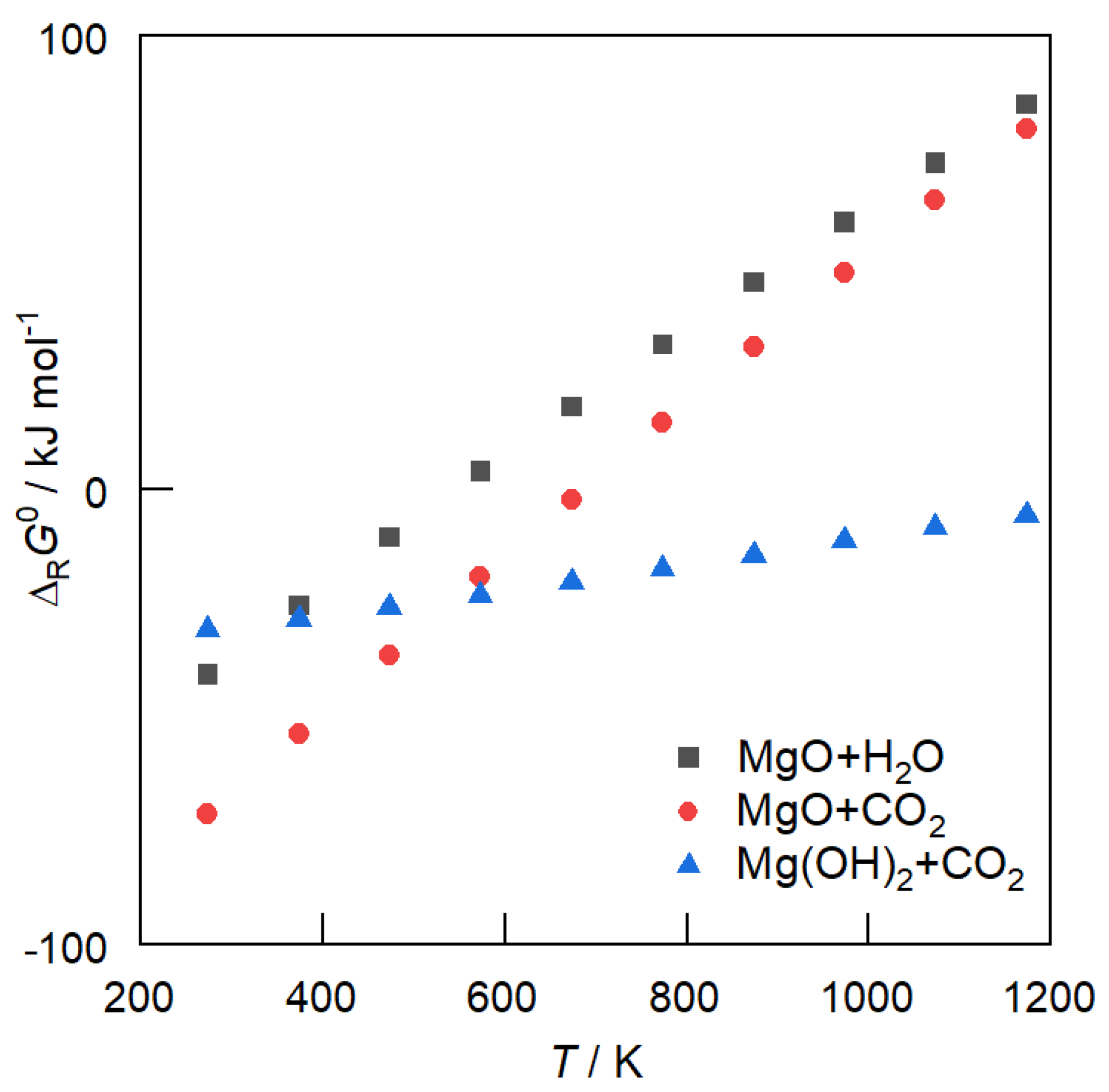
Energies, Free Full-Text

3) 40 y 38. What will the percentage purity of CaCO3 200 g of impure CaCO3 produces 22.4 L of CO2 STP in excess of HCI CaCO3 + 2HCI → CaCl2 + CO2 + H2O (1) 25% (2) 50% (3) 75% (4) 100% in 200 of so is

Less carbon producing sustainable concrete from environmental and performance perspectives: A review - ScienceDirect
36. 1.25 g of sample of limestone on heating gives 0.44 g carbon dioxide. The percentage purity of CaCO3 in limestone is

400 g sample of limestone liberates 44 g carbon dioxide on heating. The p..

58. 50 g of a sample of limestone (CaCO3) on complete 58 decomposition gives 20 g of CO2. The percentage purity of CaCO3 in limestone is (Atomic mass of Ca = 40 u) (1) 75% (2) 85% (3) 95.2% (4) 90.9% 0

Decarbonization

new developments in the generation of controlled atmospheres
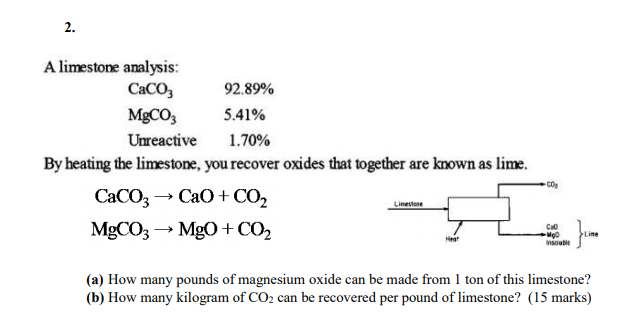
Solved A limestone analysis: CaCO, 92.89% MgCO3 5.41%
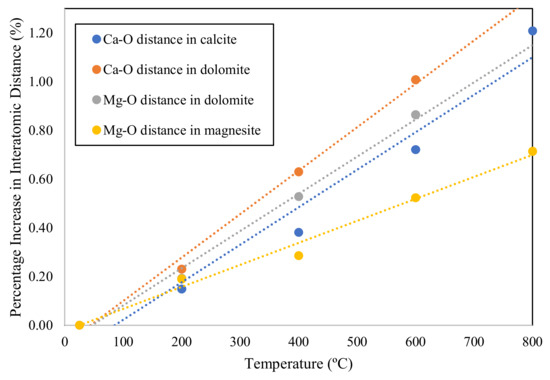
Applied Sciences, Free Full-Text
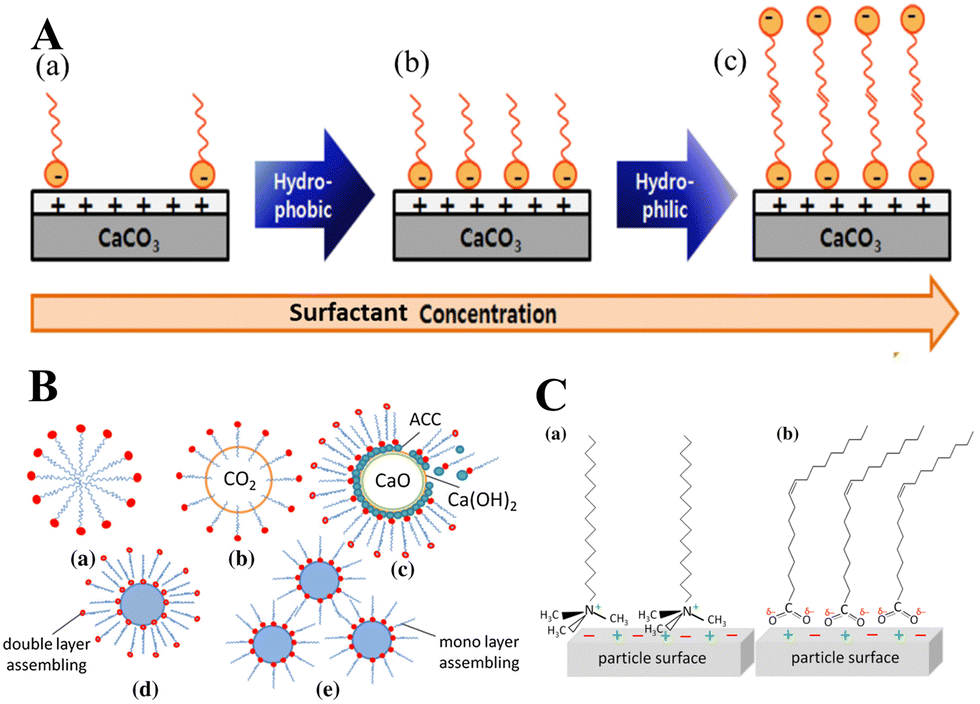
Calcium carbonate: controlled synthesis, surface functionalization, and nanostructured materials - Chemical Society Reviews (RSC Publishing) DOI:10.1039/D1CS00519G
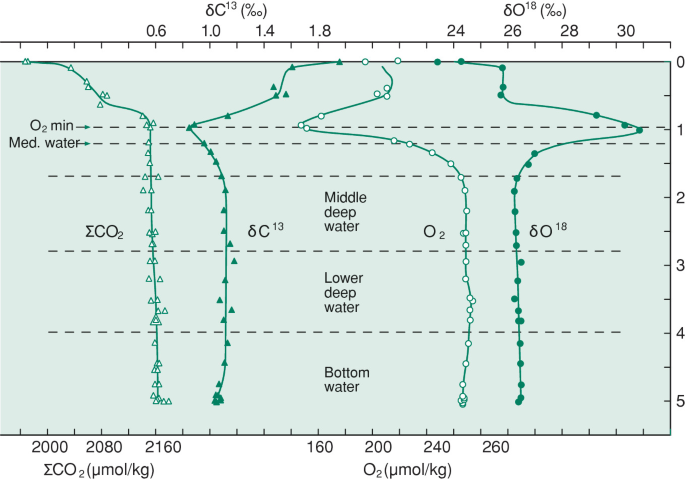
Variations of Stable Isotope Ratios in Nature

Wu unswer the questions given below it: 150 ml of N HCI is required to react completely with 1.0 g of a sample of limestone. Calculate the percentage purity of CaCO3. (A)