SOLVED: A 25 gram piece of hot metal at 97°C is plunged into a 35
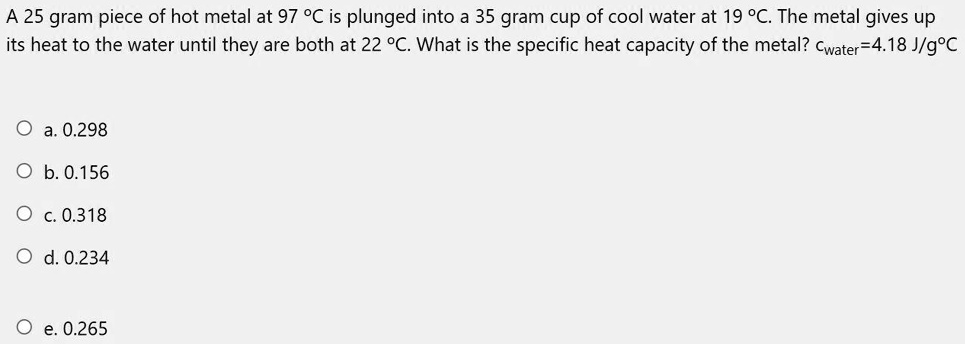

Answered: 9. A piece of iron (mass 25.0 g) at 398…
A 30 g piece of metal at 90ºC is placed in a calorimeter containing 50.0 g of water at 22.5ºC. The calorimeter itself has a heat capacity of 3.40 J/°C, and the

Lockheed Martin F-35 Lightning II - Wikipedia

Answered: Answer in joules and calories. Energy…
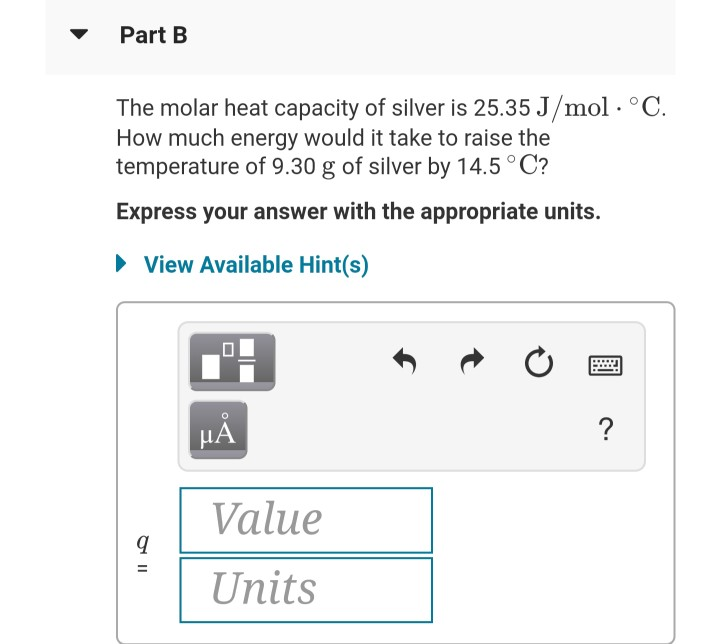
Solved Part A It takes 46.0 J to raise the temperature of an

Specific Heat Capacity

3. Tega A piece of metal weights 45 g in air and 25 g in a liquid of density 1.5 x 103 kg-m-3 kept 30°C. When the temperature of the liquid is

Solved A 55.7 -g piece of copper at 95.0 °C was
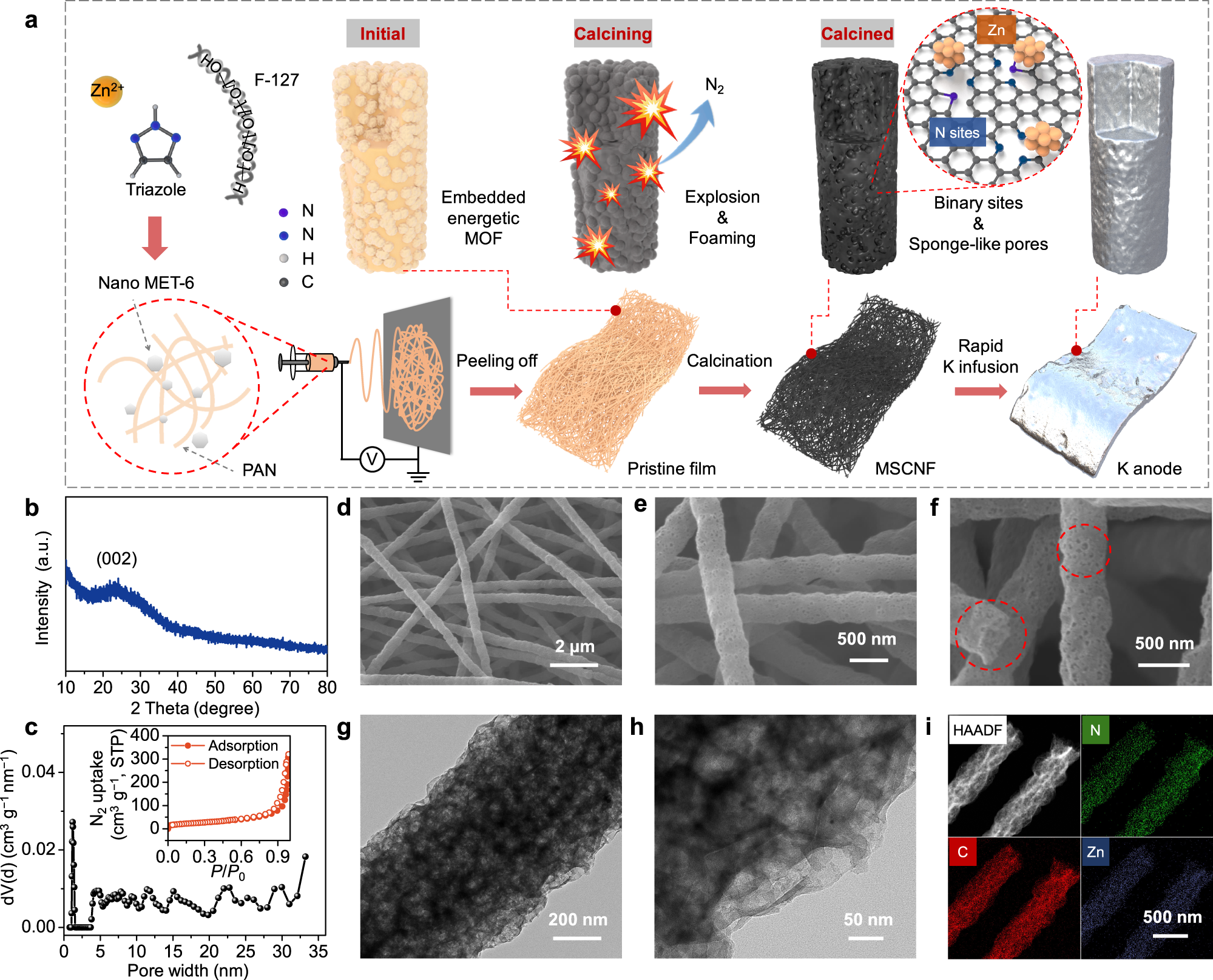
Codoped porous carbon nanofibres as a potassium metal host for

Solved 1. Calculate the amout of heat water absorbs from a
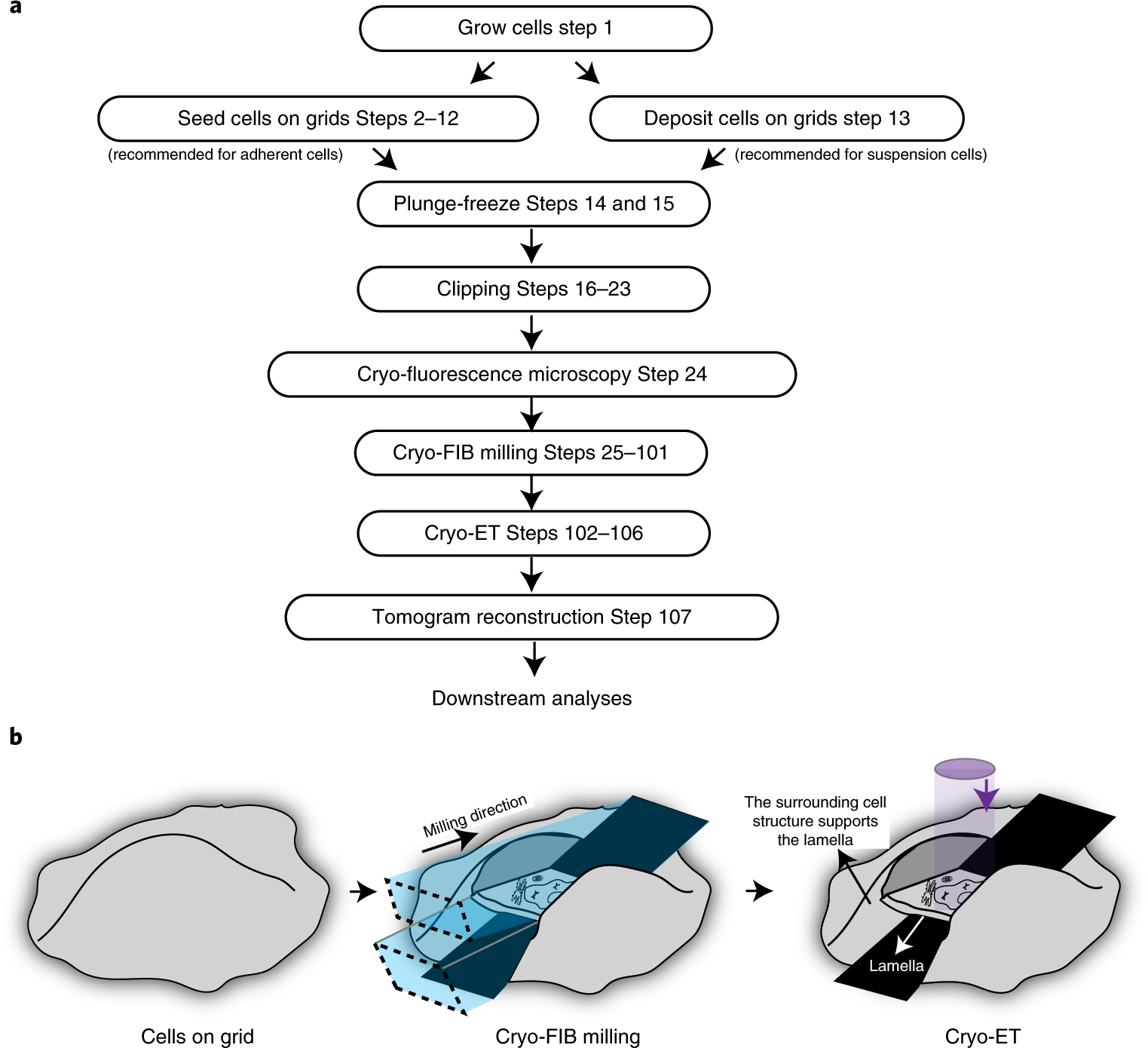
Preparing samples from whole cells using focused-ion-beam milling
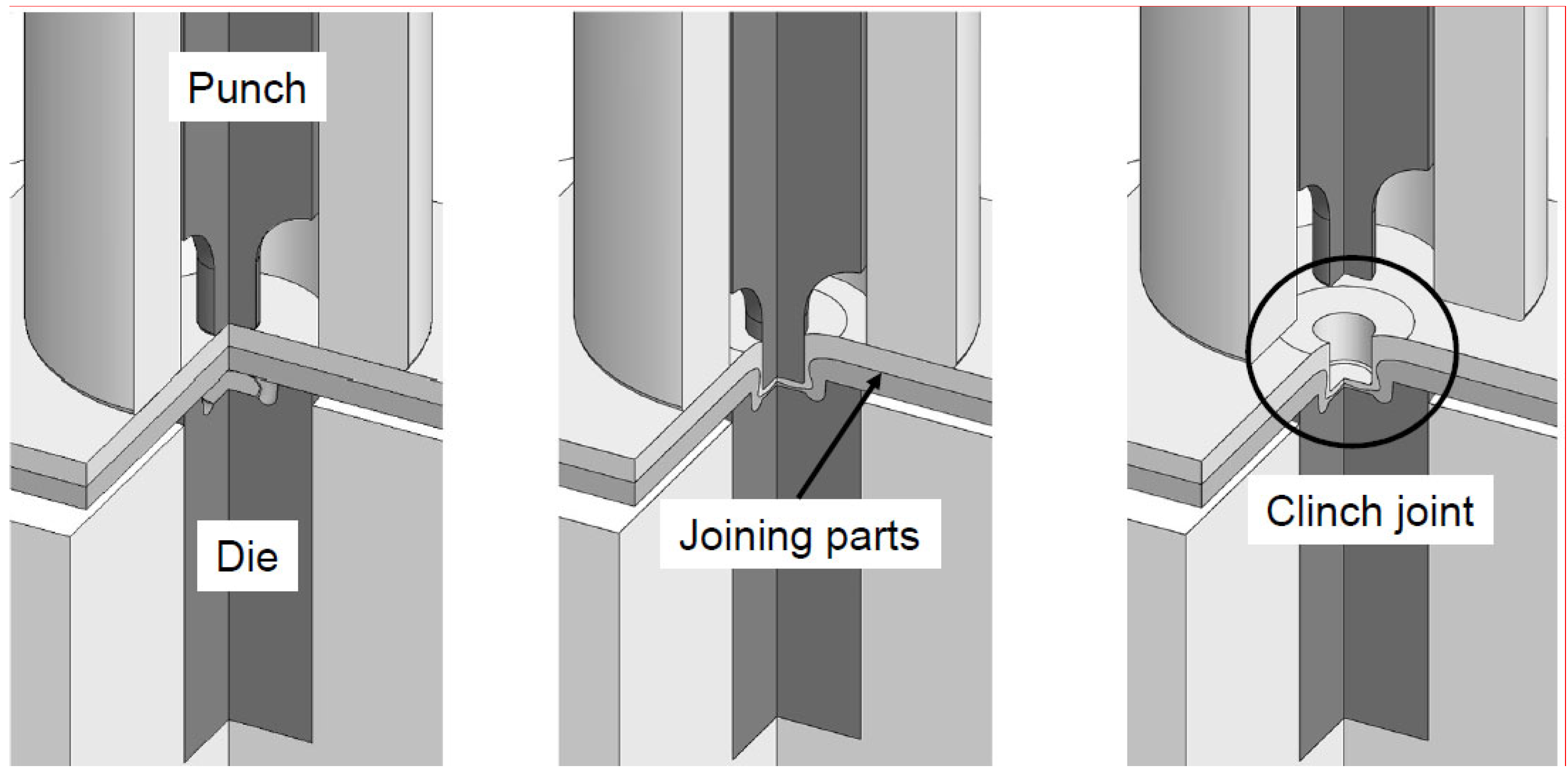
Metals, Free Full-Text

RSC Classic Chemistry Demonstrations - Royal Society of Chemistry