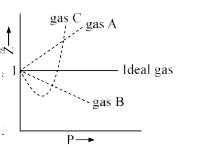
The given graph represent the variations of Z Compressibility factor Z PV nRT versus p for three real gases A B and C Identify the only incorrect statement


Gas C is a real gas and we can find 'a' and 'b' if intersection data i

The given graph represents the variations of compressibility factor `Z=PV// nRT` vs `
The given graph represent the variation of z compressibility factor z=pv/nRT versis p fpr three real gases A,B,C identify only incorrect statement
The given graph represent the variations of compressibility factor (z) = pV/nRT versus p, - Sarthaks eConnect

Van der Waals equation - Wikipedia

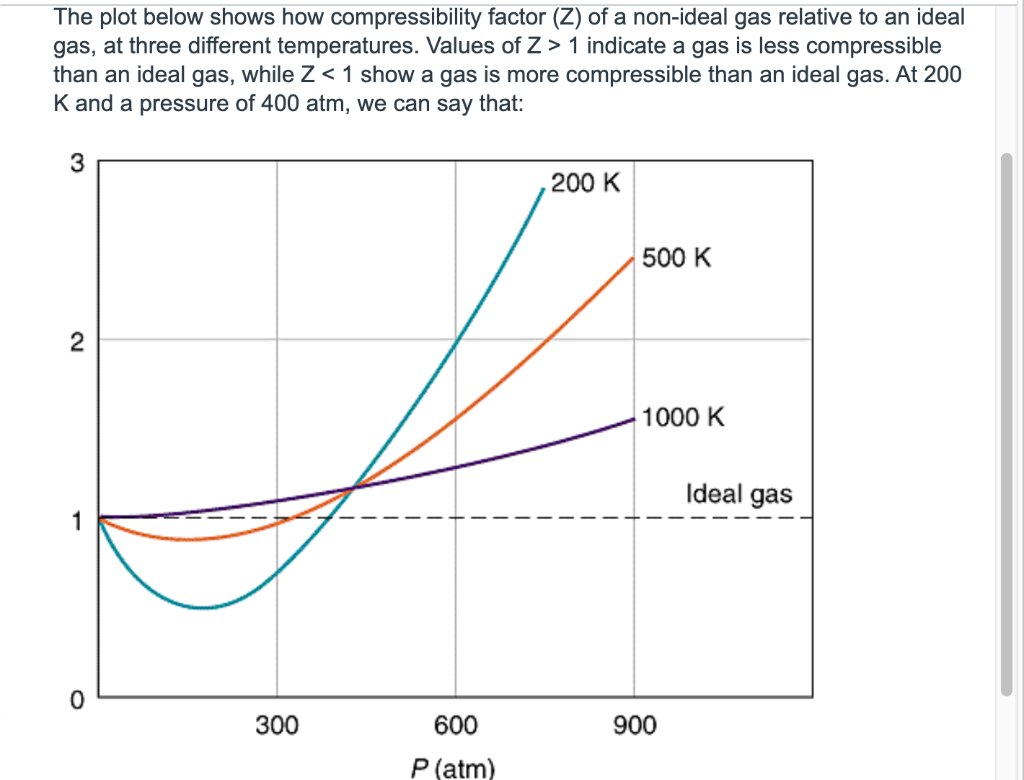
thermodynamics - Variation of compressiblity factor with temperature - Chemistry Stack Exchange

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange
Gas C is a real gas and we can find 'a' and 'b' if intersection data i

The given graph represents the variation of compressibility factor Z vs P for three gases A, B and C.Identify the incorrect statements.

the given graph represents the variation of Z (compressibility factor =dfrac{PV}{nRT}) versus P, three real gases A, B and C. Identify the only correct statement.For the gas A, a = 0 and

Compressibility factor - Wikipedia

Real Gases, PDF, Gases
Solved Using the Maxwell-Boltzmann distribution curves

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application
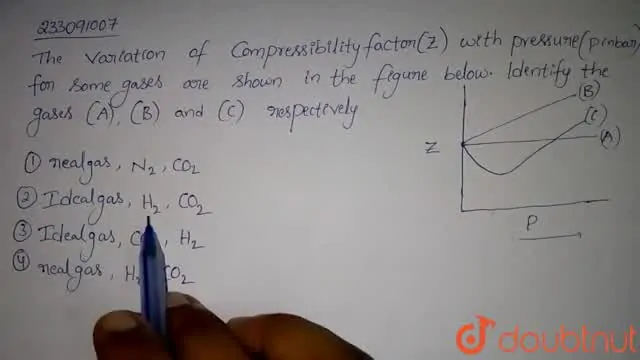
Telugu] The variation of compressibility factor (Z) with pressure (p