physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate. In
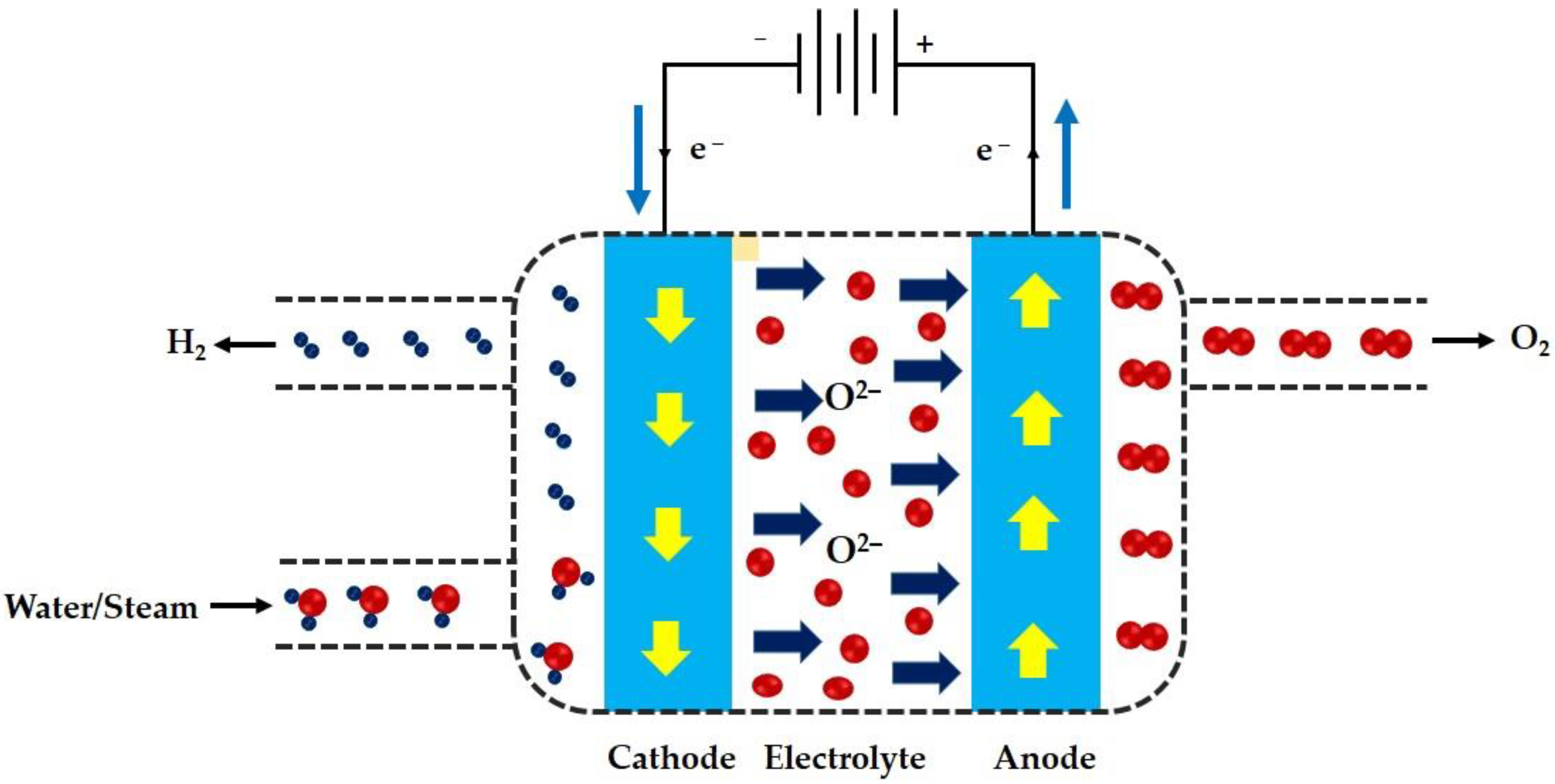
Energies, Free Full-Text

physical chemistry - Pressure vs volume plot for real gas and

COMPRESSIBILITY factor Z, Using P and v in 3 Minutes!

Compressibility Factor - an overview

physical chemistry - why is the pressure exerted by ideal gas on

Effects of mechanical pressure on anion exchange membrane water
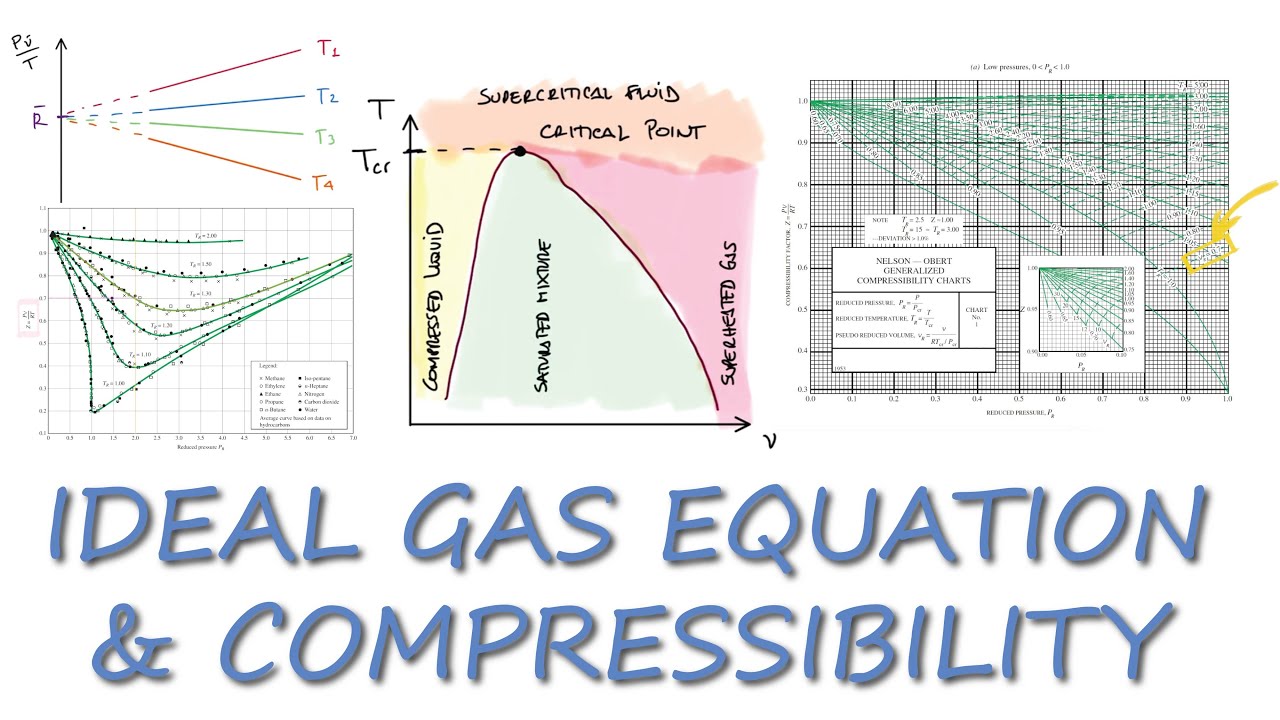
Ideal Gas Equation and COMPRESSIBILITY Factor in 11 Minutes!

Heat pump - Wikipedia
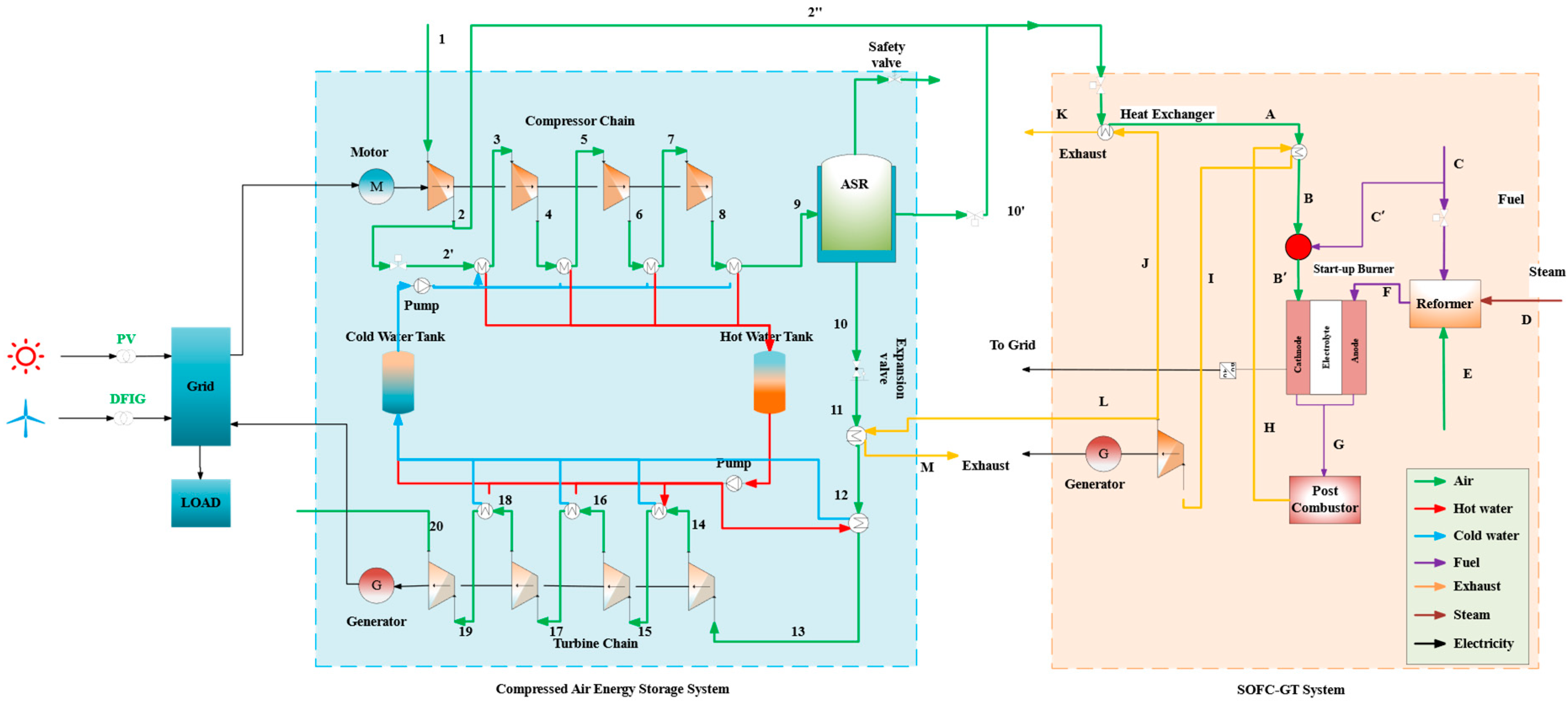
Energies, Free Full-Text

Strain engineering of two‐dimensional materials: Methods
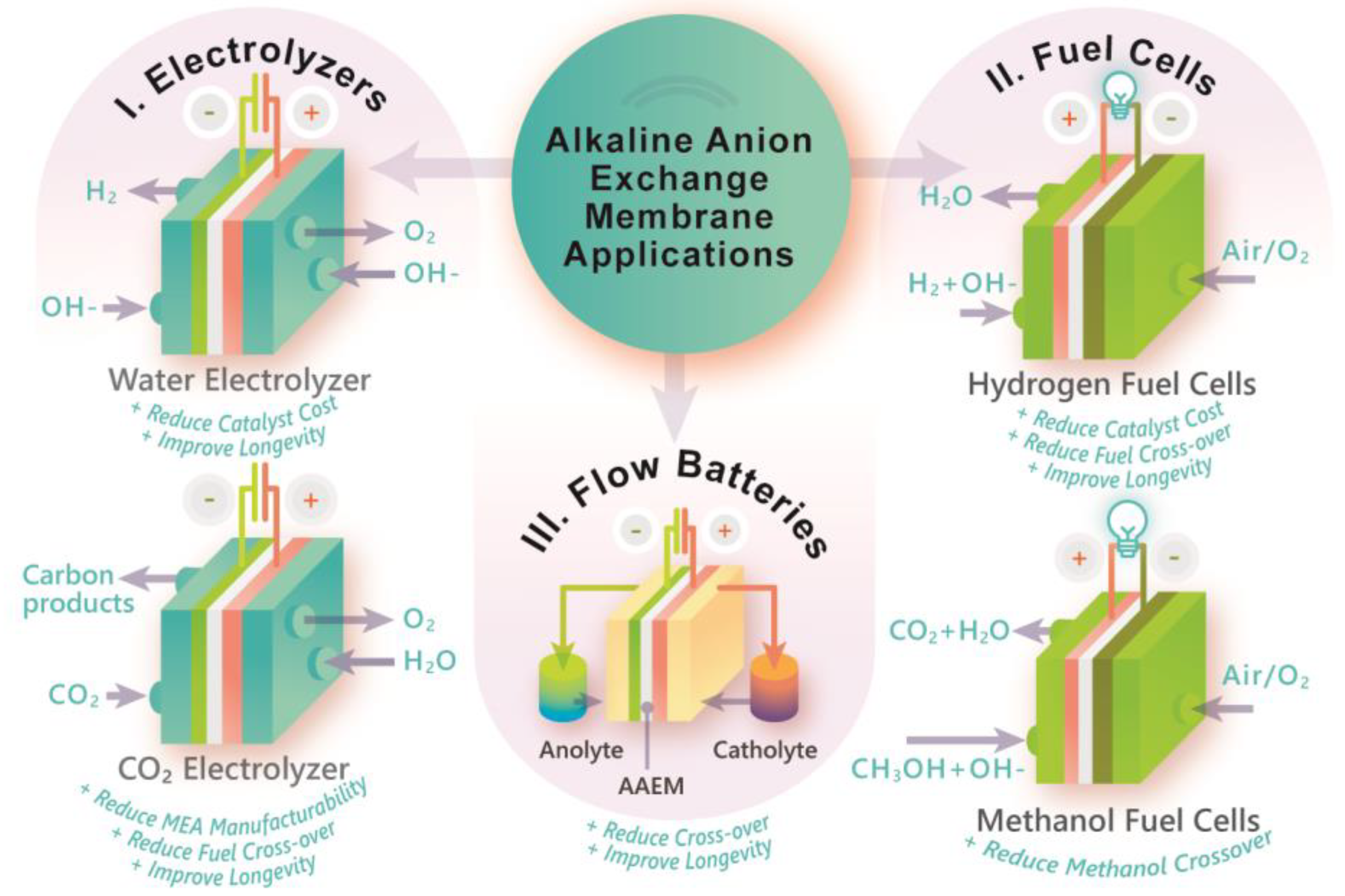
Polymers, Free Full-Text