At low pressure, the van der waal's equation is written as (P+ a/V
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to :
At low pressure- the van der waal-s equation is written as -P- a-V-2-V-RT - Then compressibility factor is then equal to

Solved 3. Consider the Van der Waals equation of state (P+
How I find the a and b constant in the Van der Waals equation? - Quora

Q.4 The van der Waals' equation for a gas is (p+V2a)(V−b)=nRT

REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR
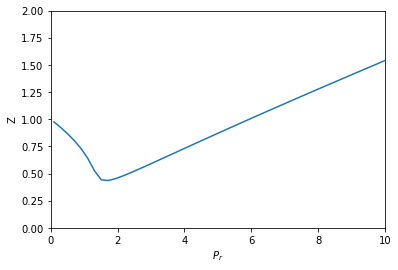
Compressibility factor variation from the van der Waals equation
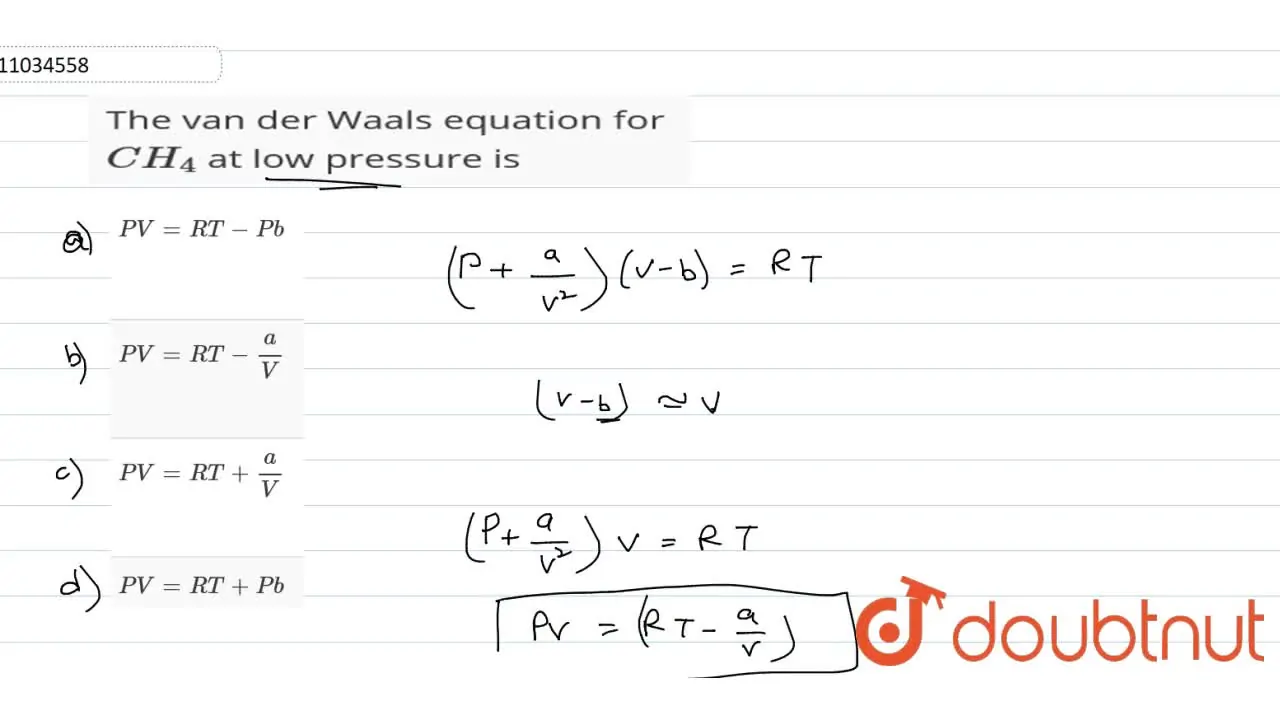
The van der Waals equation for CH(4) at low pressure is

Solved The Van der Waal's equation of state is: P = nRT/V

The van der Waals equation gives a relationship between the
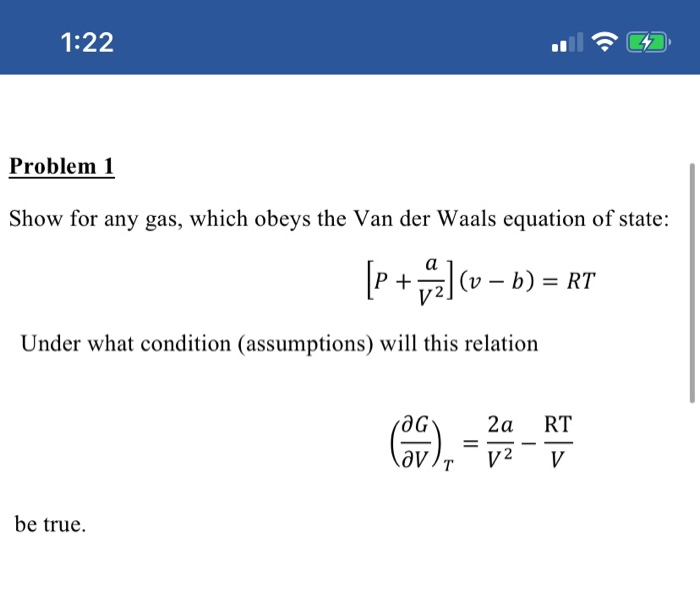
Solved show for any gas, which obeys the Van der Waals

Rearrange the van der Waals equation of state $p=n R T /(V-n
Van der Waals equation (gas law for real gases) - tec-science

The van der waals equation for a gas is (P + a/v2)(V-b) = RT where