The FDA's rule change requiring providers to inform women about

The FDA Needs More Power to Regulate Toxic Chemicals in Cosmetics

Time to Refresh? FDA Issues Draft Guidance on Key Information and Informed Consent
Essential Screenings for Women – ActiveBeat – Your Daily Dose of

To Fight Childhood Obesity, Tackle Maternal Obesity - UPMC & Pitt

Adrian Waller on LinkedIn: #finditearly #breastcancer

Therapy With MDMA Can Help Treat PTSD, Will the Drug Get FDA Approval?

Cancer can go undetected in dense breasts. A new FDA rule will require providers to inform patients of that risk

Opill: The FDA approved the US' first over-the-counter birth control pill. What happens next?
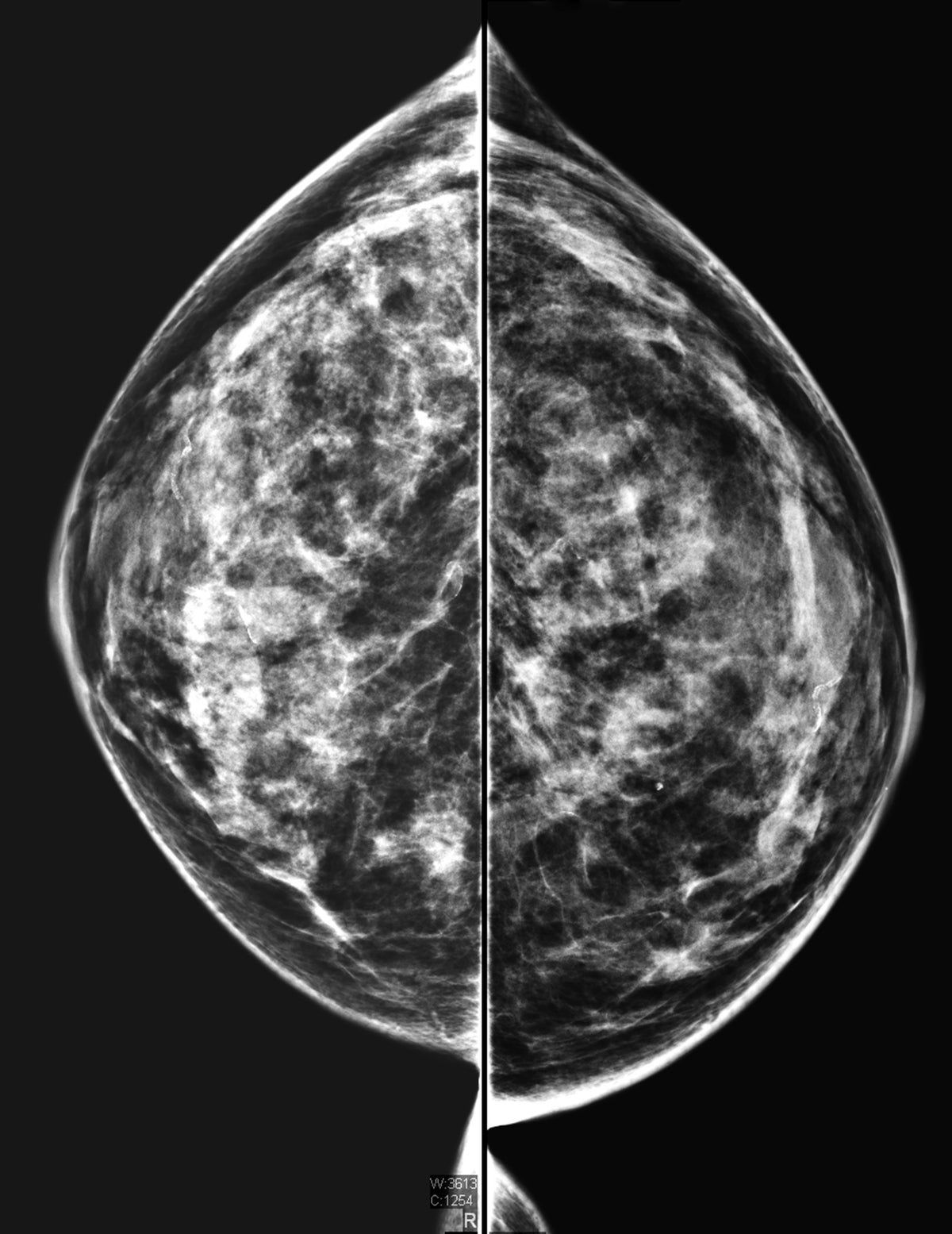
What the FDA Ruling about 'Dense Breasts' Means for Cancer Risk and Screening