What is the change in internal energy (in J) of a system that

I found an increase of 3100J Have a look
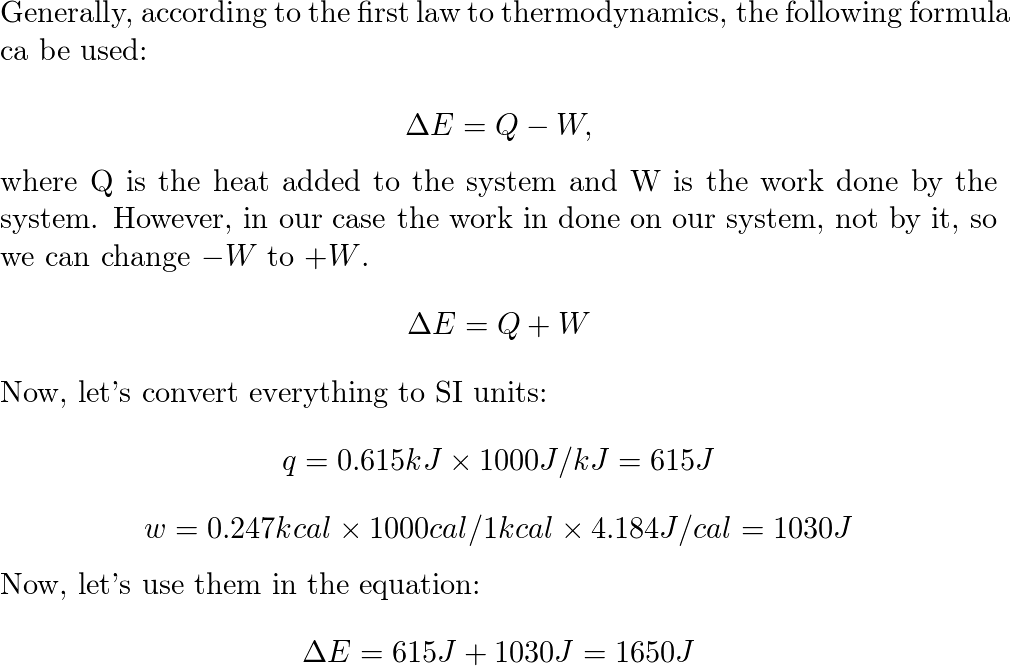
What is the change in internal energy (in J) of a system tha

Using the First Law of Thermodynamics to Calculate Work Done, Physics
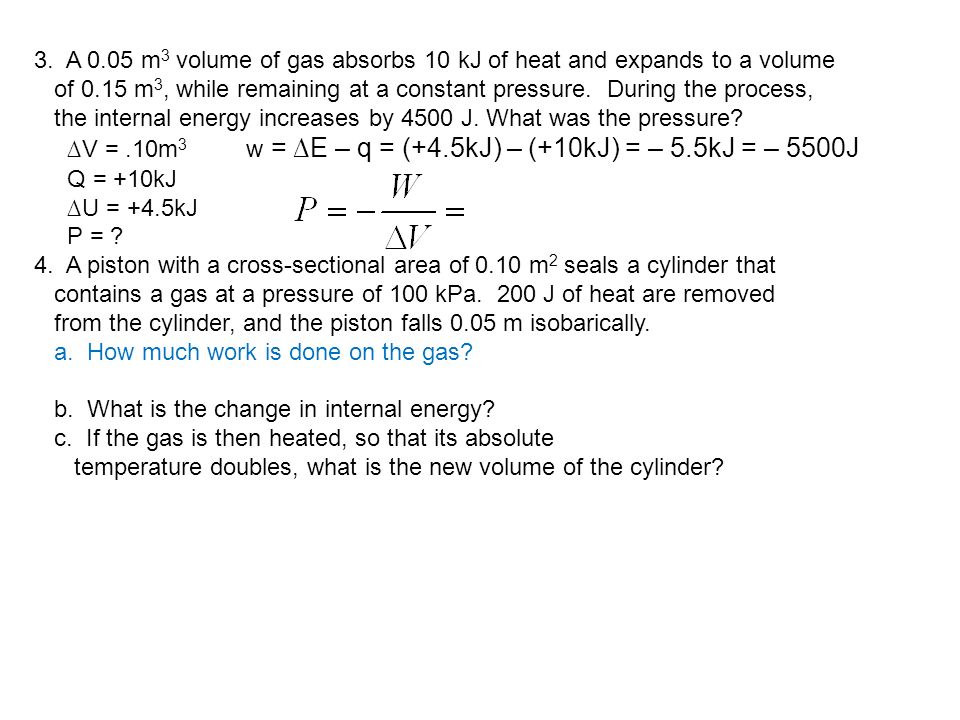
Ch6.1 The Nature of Energy (hustle!) - ppt download

Solved Be sure to answer all parts. What is the change in
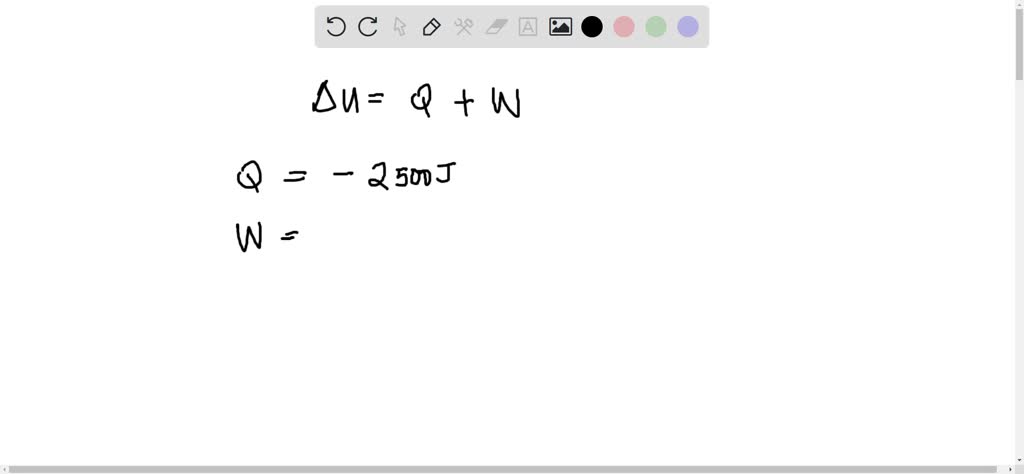
SOLVED: The change in the internal energy of a system that releases 2,500 J of heat and that does 7,655 J of work on the surroundings is J.
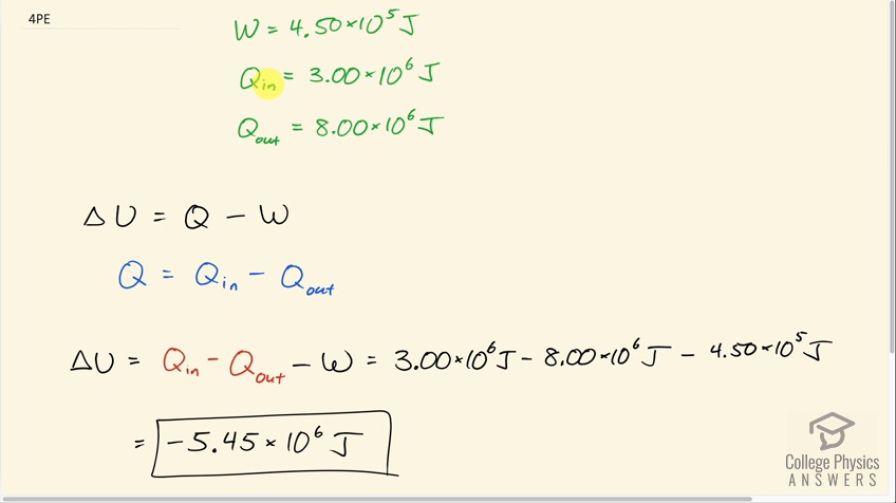
OpenStax College Physics, Chapter 15, Problem 4 (Problems & Exercises)

A system absorbs 196 kJ of heat and the surroundings do 117 kJ of
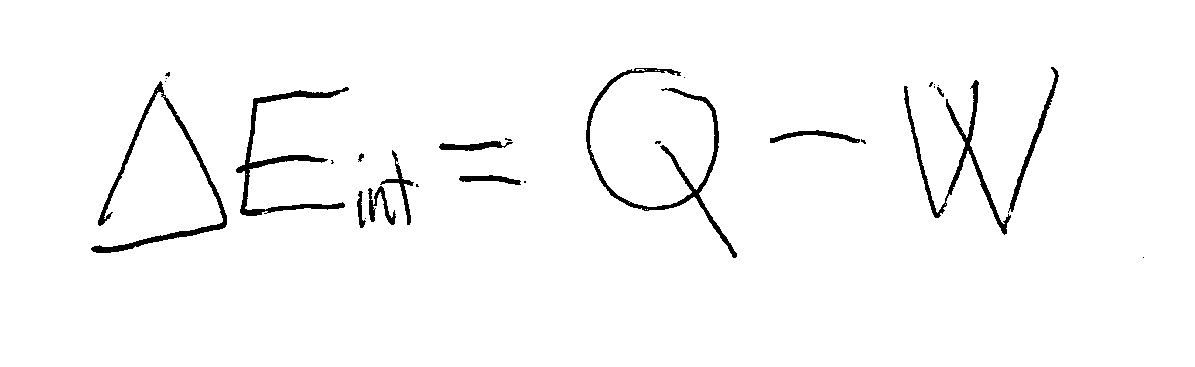
Heat and Work - Physics
Change in Internal Energy Calculator - Calculator Academy
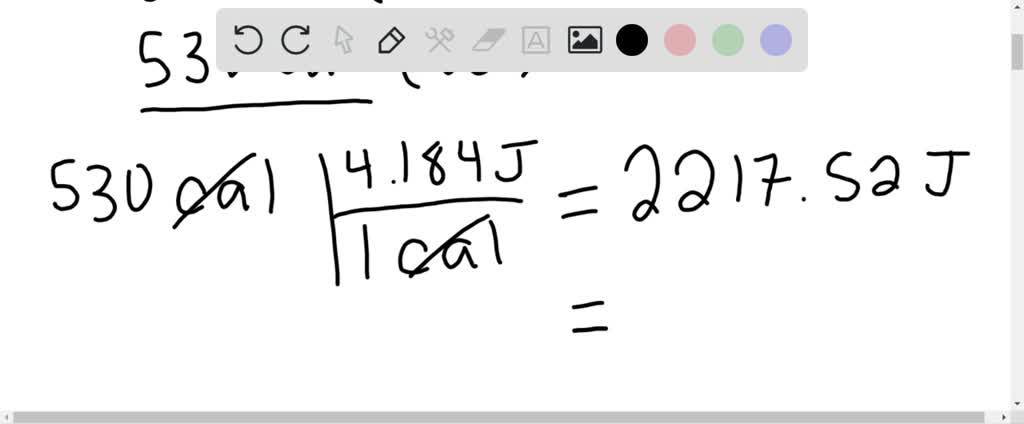
⏩SOLVED:What is the change in internal energy (in J) of a system

⏩SOLVED:A system takes in 550 J of heat while performing 840 J of