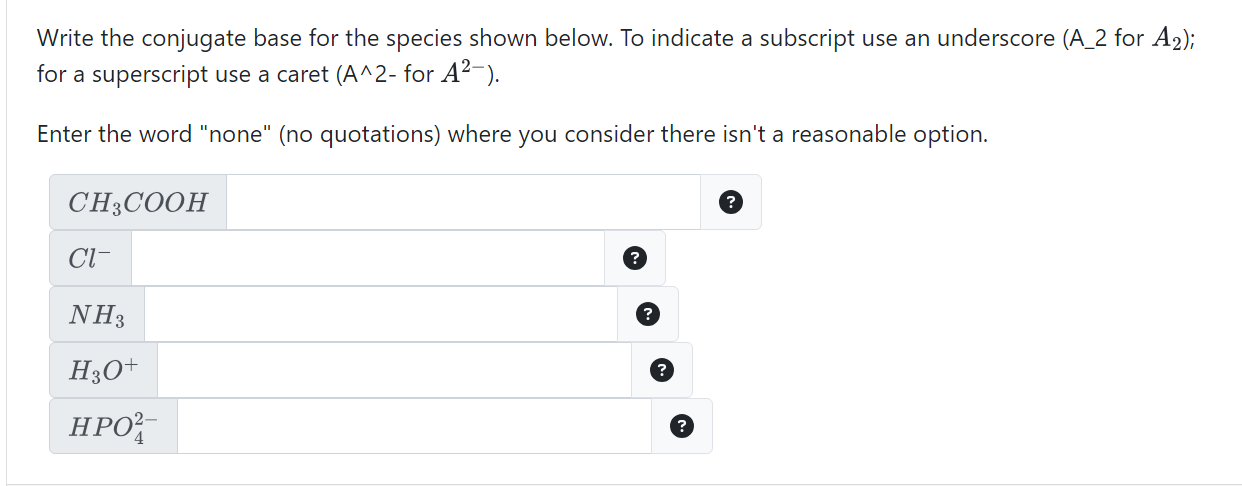
Solved Write the conjugate base for the species shown below
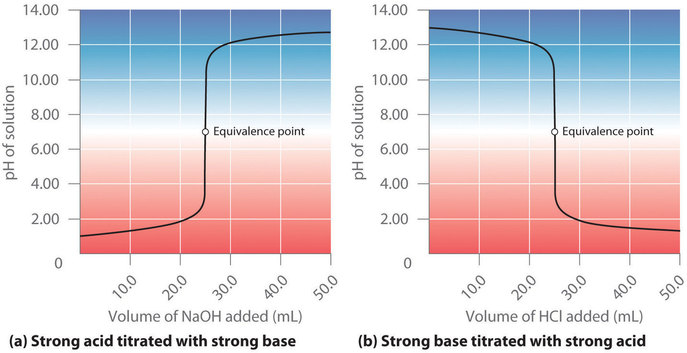
15.6: Acid-Base Titration Curves - Chemistry LibreTexts
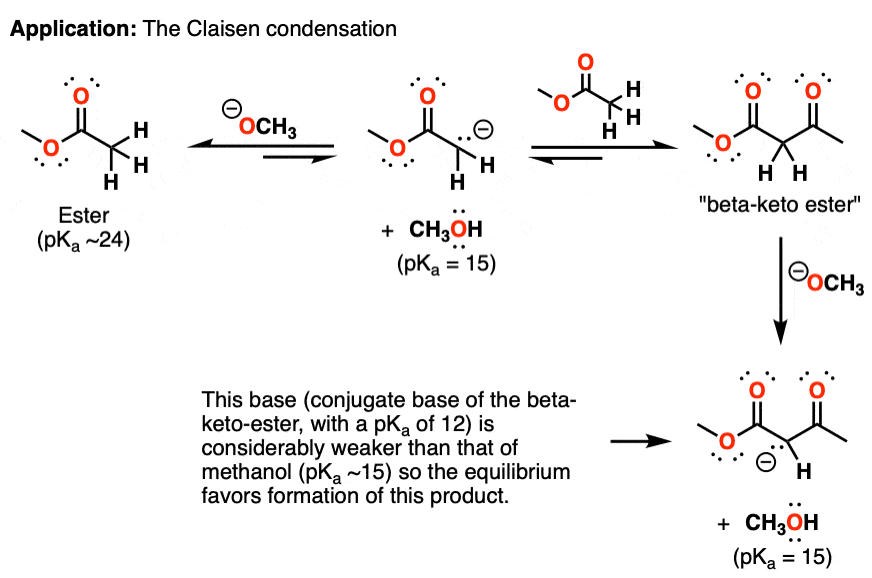
Reversible and Irreversible Acid-Base Reactions In Organic Chemistry

The following diagrams represent aqueous solutions of three acids

The species: H2O, HCO3-,HSO4- and NH3 can act both as Bronsted acids and bases..

Solved Write the conjugate acid of the following species

SOLVED: Write under each species shown – acid /or base and that of their conjugate acid / base pairs in the following reactions. (2X2= 4) (SLO) a) NO2- + HCO3- CO3-2 +
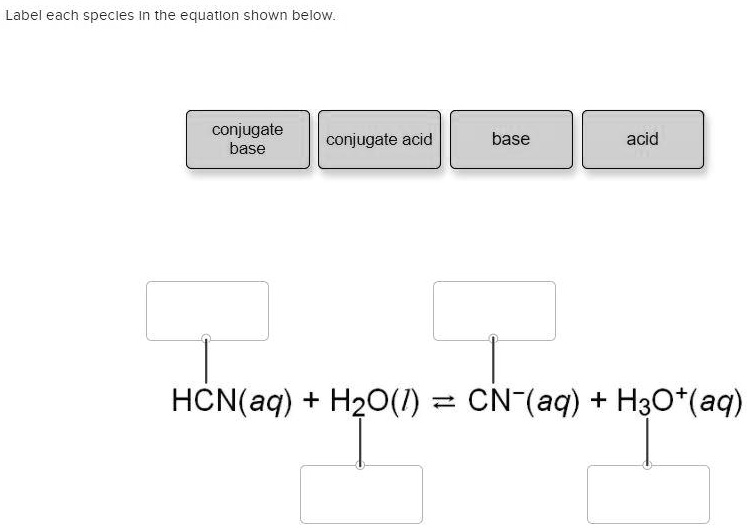
SOLVED: Label each species in the equation shown below: conjugate base conjugate acid base acid HCN(aq) + H2O(l) = CN-(aq) + H3O+(aq)
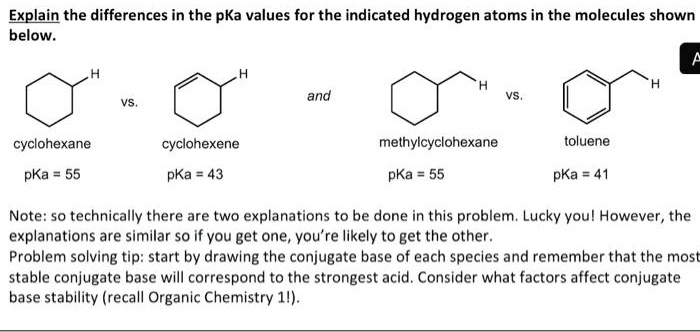
SOLVED: Explain the differences in the pKa values for the indicated hydrogen atoms in the molecules shown below: and cyclohexane cyclohexene methylcyclohexane toluene pKa pKa 43 pKa pKa Note: So technically there

In the Acid-Base reaction shown below, write the structure of the conjugate base of tropolone. Using curved arrow notation write down all possible resonance structures for this conjugate base.
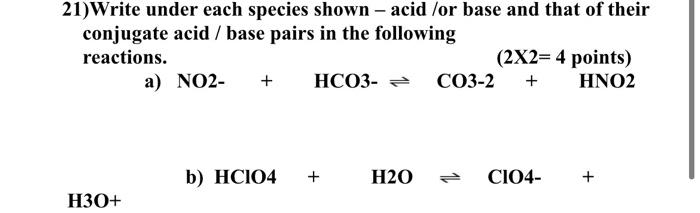
Solved 21)Write under each species shown - acid /or base and
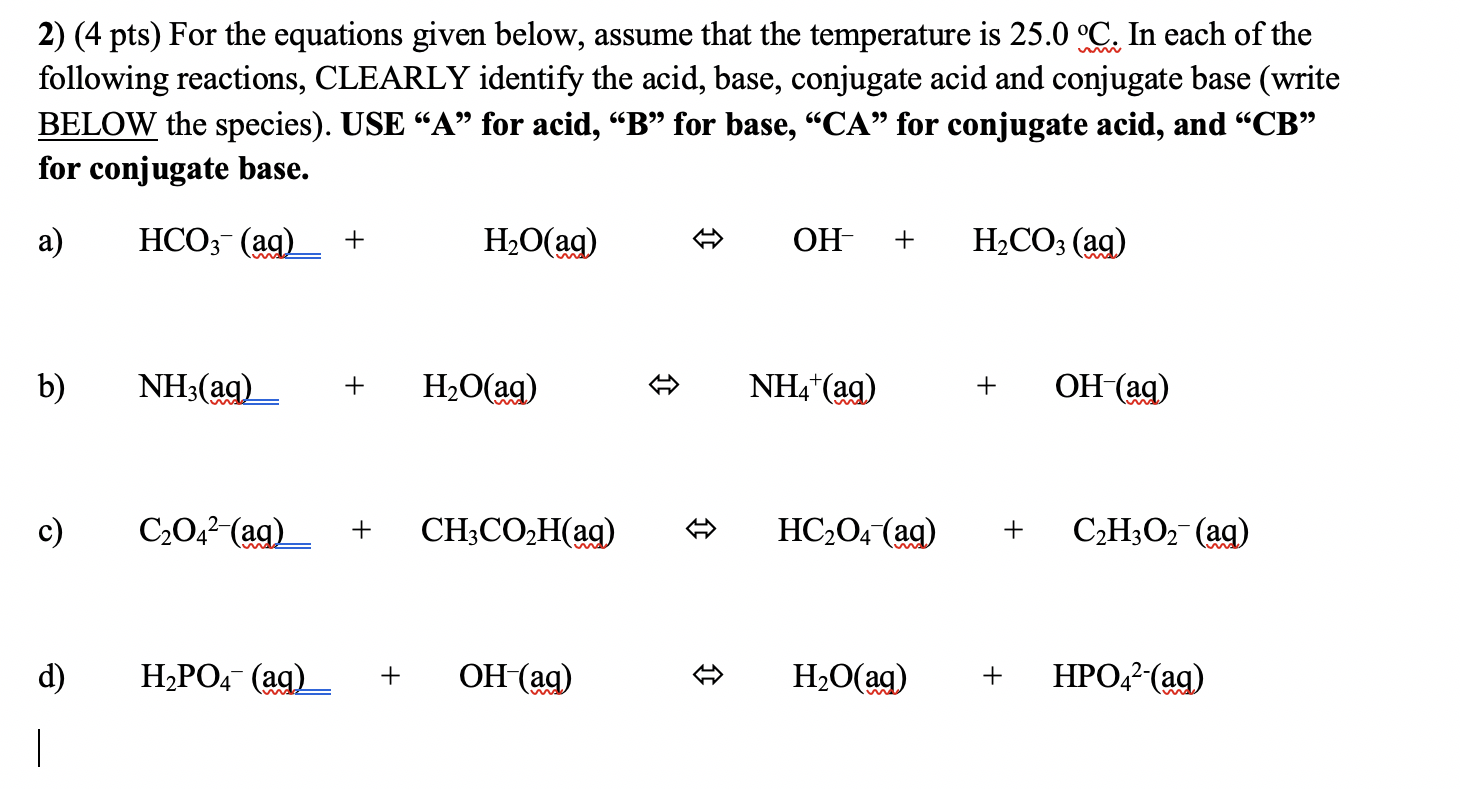
Solved 2) (4 pts) For the equations given below, assume that
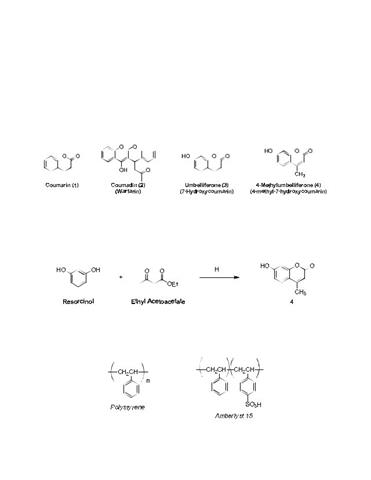
Solved) - (Part of post lab questions 1, 2, and 9) I need help drawing all (1 Answer)