What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons-an t at different conditions of pressure and volume-Why is Z-1 for H2 and He gas

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

compressible flow related terms - Department of Mechanical and

Solved Real gas effects can be expressed as departures from

Torateal gas, the compressibility factor Z has different whues different temperatures and pressures. Which of the following is not correct under the given conditions? (a) Z<1 very low pressure. (b) Z>1 high
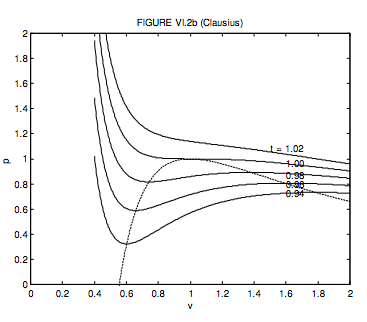
6.3: Van der Waals and Other Gases - Physics LibreTexts

Fluids, Free Full-Text
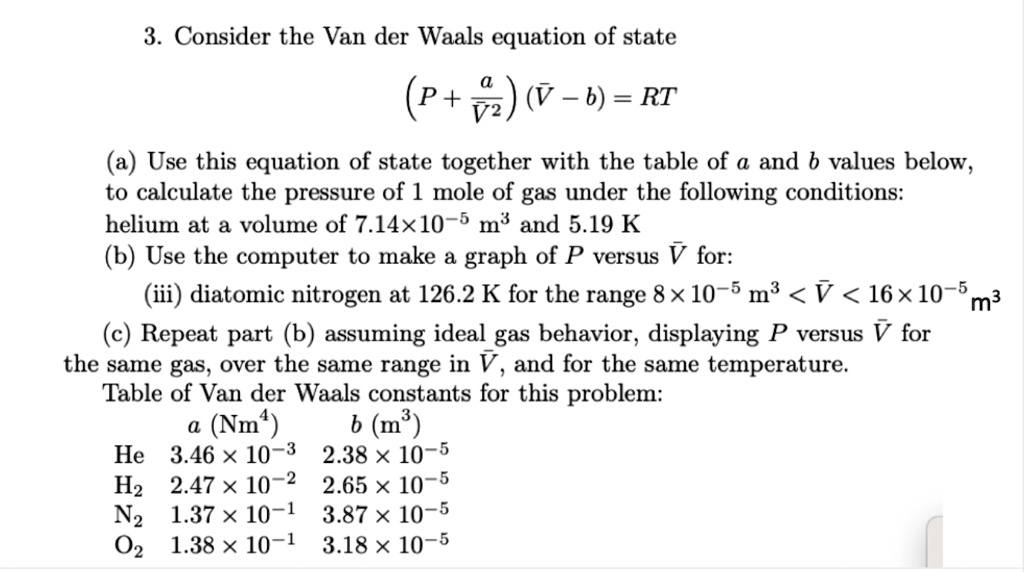
SOLVED: Consider the Van der Waals equation of state (P + a/V^2)(V - b) = RT (a) Use this equation of state together with the table of a and b values below
Slope of graph of compressibility factor(Z) with pressure(P) for hydrogen gas at any pressure i

1.7: Connecting the van der Waals and the viral equations- the Boyle temperature - Chemistry LibreTexts
How I find the a and b constant in the Van der Waals equation? - Quora